Uranium 235 Nuclear Fission Reaction
Uranium 235 Nuclear Fission Reaction, Indeed recently has been hunted by consumers around us, perhaps one of you personally. People now are accustomed to using the internet in gadgets to view video and image information for inspiration, and according to the name of this article I will discuss about
If the posting of this site is beneficial to our suport by spreading article posts of this site to social media marketing accounts which you have such as for example Facebook, Instagram and others or can also bookmark this blog page.
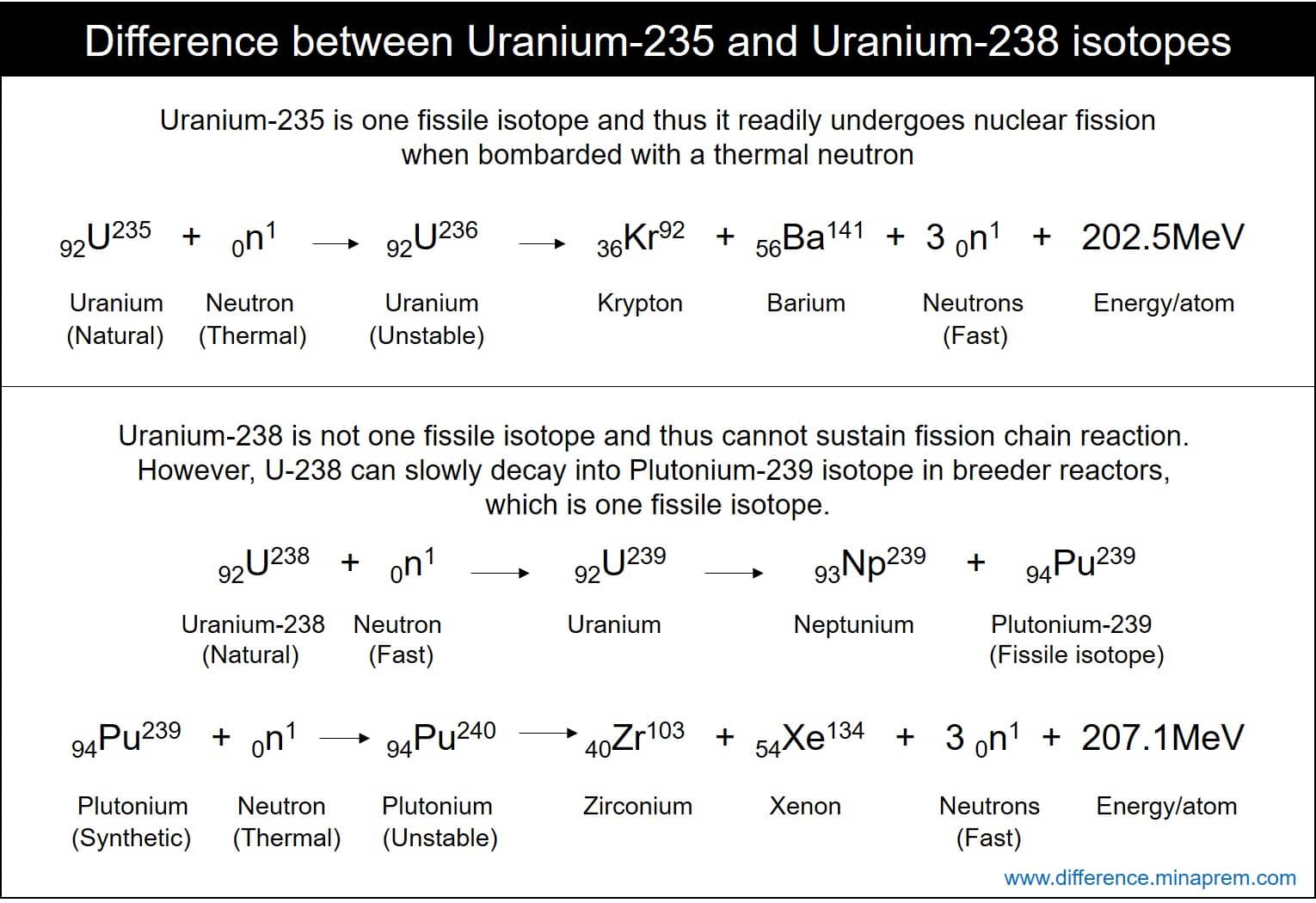
Uranium 235 235 u is an isotope of uranium making up about 072 of natural uraniumunlike the predominant isotope uranium 238 it is fissile ie it can sustain a fission chain reactionit is the only fissile isotope that exists in nature as a primordial nuclide.
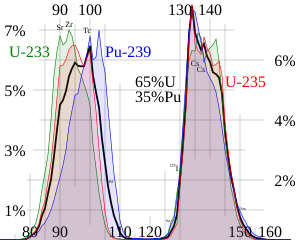
Liquid glass nuclear waste. In most nuclear fission examples and exercises the products of a nuclear fission of uranium 235 are two light nuclei of krypton and barium. The cross section for radiative capture for thermal neutrons. Hence uranium is widely used in nuclear experiments.
U 235 decays naturally by alpha radiation. Table 13 fission produces large nuclear fragments. The cross section for radiative capture for thermal neutrons is about 99 barns for.
Uranium 235 is a fissile isotope and its fission cross section for thermal neutrons is about 585 barns for 00253 ev neutron. Each time a u 235 nucleus splits it releases two or three neutrons. Uranium is a common element on earth and has existed since the planet formed.
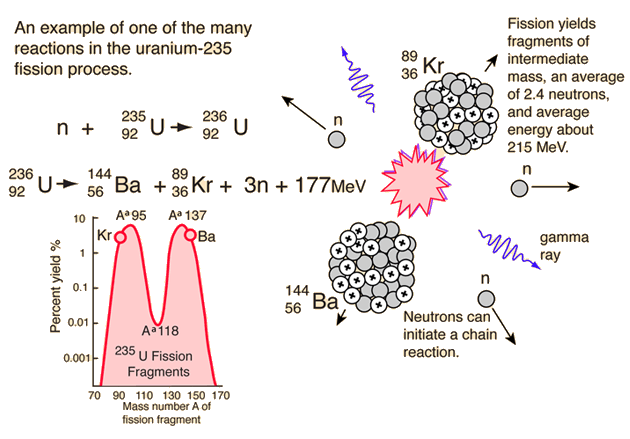
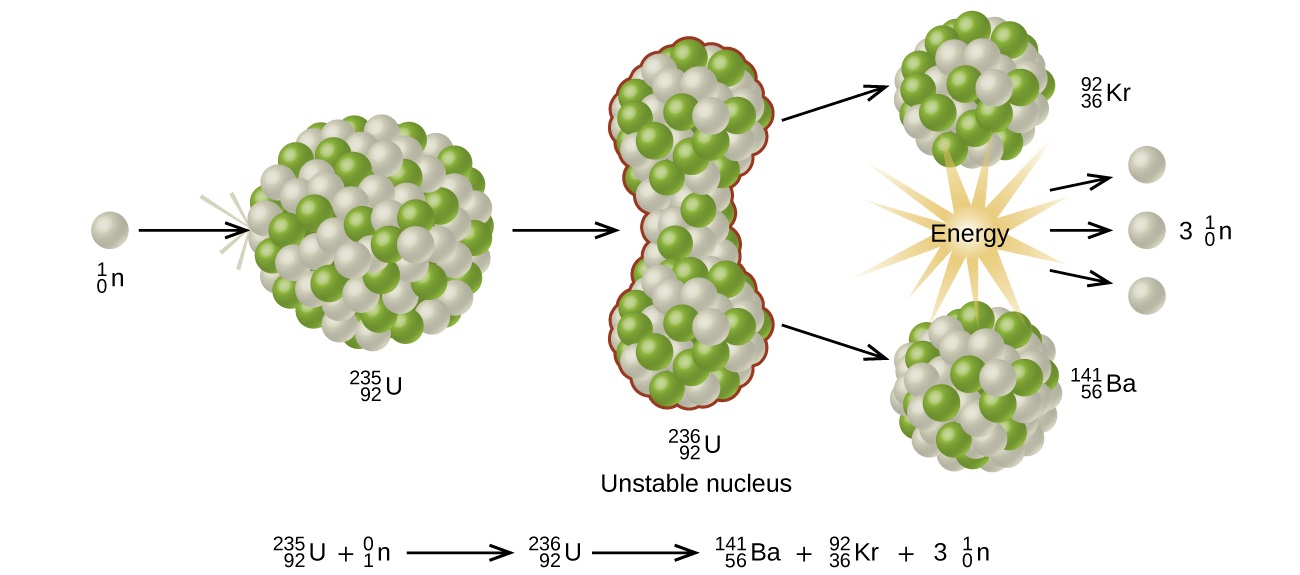
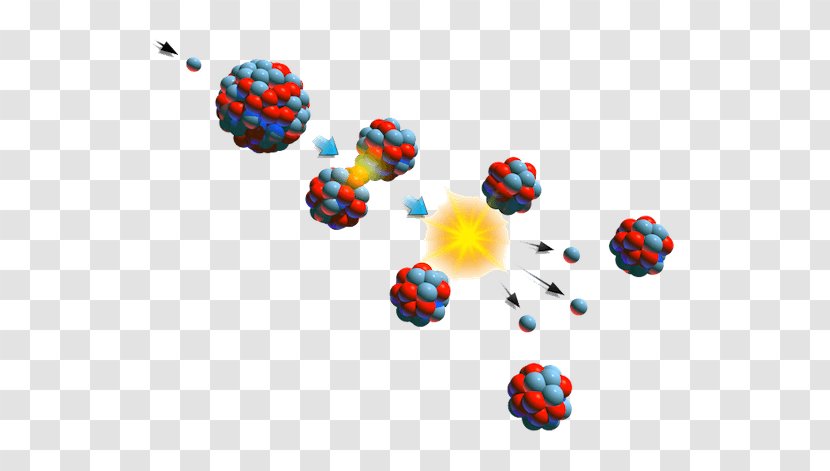
Uranium 235 is the only naturally occurring fissile nuclide. Mathrm 01n u longmapsto kr ba energy is there some fission reaction that produces more stable nuclei instead of krypton and barium. Uranium 235 chain reaction if an least one neutron from u 235 fission strikes another nucleus and causes it to fission then the chain reaction will continue.
When a u 235 nucleus absorbs an extra neutron it quickly breaks into two parts. It was discovered in 1935 by arthur jeffrey dempster. If the reaction will sustain itself it is said to be critical and the mass of u 235 required to produced the critical condition is said to be a critical mass.
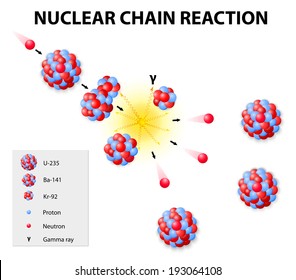
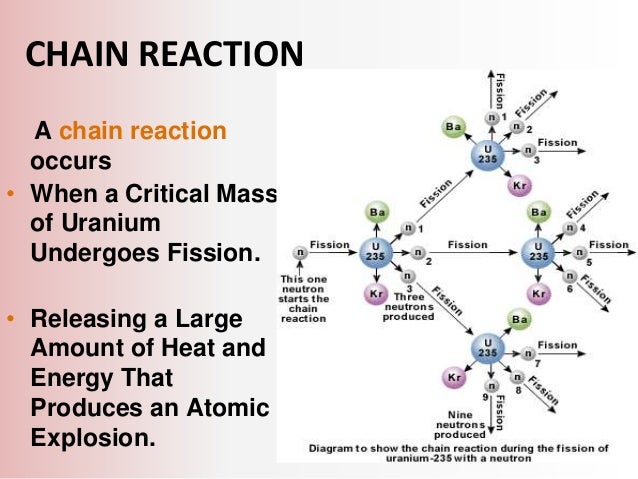
For fast neutrons its fission cross section is on the order of barnsmost of absorption reactions result in fission reaction but a minority results in radiative capture forming 236 u. If enough of these expelled neutrons cause the nuclei of other u 235 atoms to split releasing further neutrons a fission chain reaction can be achieved. The fuel most widely used by nuclear plants for nuclear fission is uranium.
It is a common metal found in rocks. This process is known as fission see diagram below. Please mention all possible fission equations of uranium.
13 fissile nuclides undergo thermal fission stimulated by neutron capture. The specific nuclear reaction may be the fission of heavy isotopes eg uranium 235 235 u. The nuclear chain reaction releases several million times more energy per reaction than any.
Nuclear chain reaction occurs when one single nuclear reaction causes an average of one or more subsequent nuclear reactions thus leading to the possibility of a self propagating series of these reactions. For fast neutrons its fission cross section is on the order of barnsmost of absorption reactions result in fission reaction but a minority results in radiative capture forming 236 u. While there are several varieties of uranium uranium 235 u 235 is the one most important to the production of both nuclear power and nuclear bombs.
It throws off an alpha particle or two neutrons and two protons bound together. The arrangement of particles within uranium 235 is somewhat unstable and the nucleus can disintegrate if it is excited by an outside source.