Uranium 235 Nuclear Fission Chain Reaction
Uranium 235 Nuclear Fission Chain Reaction, Indeed recently has been hunted by consumers around us, perhaps one of you personally. People now are accustomed to using the internet in gadgets to view video and image information for inspiration, and according to the name of this article I will discuss about
If the posting of this site is beneficial to our suport by spreading article posts of this site to social media marketing accounts which you have such as for example Facebook, Instagram and others or can also bookmark this blog page.
The mass of uranium 235 that is required to produce a reaction that is self sustaining is said to be the critical mass.

Nuclear radiation vodka. For u 235 on average 25 neutrons are emitted starting on average two more fission reactions. Nuclear chain reaction occurs when one single nuclear reaction causes an average of one or more subsequent nuclear reactions thus leading to the possibility of a self propagating series of these reactions. 13 fissile nuclides undergo thermal fission stimulated by neutron capture.
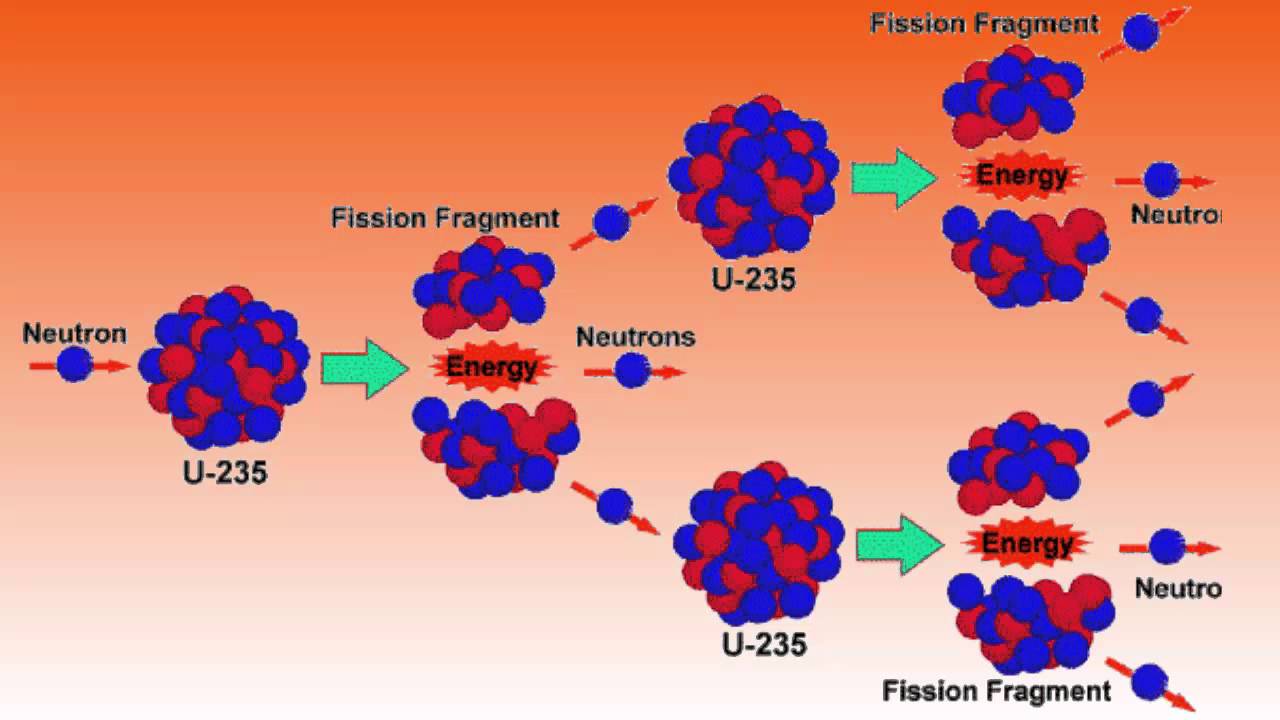
One of the most well known and useful examples of a chain reaction is of u 235 which is used to harness nuclear energy. But u 235 is the most common isotope to use for a nuclear chain reaction. This causes the nucleus to.
Uranium 235 is a fissile isotope and its fission cross section for thermal neutrons is about 585 barns for 00253 ev neutron. If you do the math this is an exponential increase. You control this by staying exactly critical when the neutrons released equal the neutrons that create a fission leak.
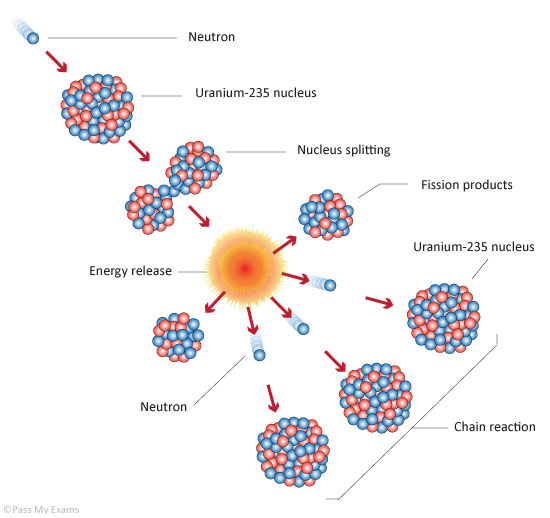
This critical chain reaction can be accomplished at relatively low uranium 235 concentrations if the neutrons are moderated to lower their speed. Chain reactions are basically fission reactions which through the products produce more chain reactions. Nuclear fission is the splitting of a large atomic nucleus into smaller nuclei.
The cross section for radiative capture for thermal neutrons. Uranium 235 has a half life of 7038 million years. Nuclear chain reaction process.
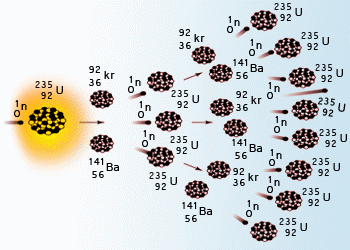
For fast neutrons its fission cross section is on the order of barnsmost of absorption reactions result in fission reaction but a minority results in radiative capture forming 236 u. In a nuclear reactor a neutron is absorbed into a nucleus typically uranium 235. If the reaction will sustain itself it is said to be critical and the mass of u 235 required to produced the critical condition is said to be a critical mass.
For every u235 atom that fissions an average of 22 neutrons are released. A moderator is necessary because the probability for fission is greater with slow neutrons. Table 13 fission produces large nuclear fragments.
Thermal fission table 13 generates two or more neutrons sufficient to sustain the nuclear chain reaction harnessed by nuclear reactors and nuclear weaponsunlike other decay reactions cf. The specific nuclear reaction may be the fission of heavy isotopes eg uranium 235 235 u. The nuclear chain reaction releases several million times more energy per reaction than any.
It was discovered in 1935 by arthur jeffrey dempster. Some other fissionable materials u 233 pu 239 are present in the world. Uranium 235 235 u is an isotope of uranium making up about 072 of natural uraniumunlike the predominant isotope uranium 238 it is fissile ie it can sustain a fission chain reactionit is the only fissile isotope that exists in nature as a primordial nuclide.
Below is a simple fission process.