Alpha Radiation Nuclear Symbol
Alpha Radiation Nuclear Symbol, Indeed recently has been hunted by consumers around us, perhaps one of you personally. People now are accustomed to using the internet in gadgets to view video and image information for inspiration, and according to the name of this article I will discuss about
If the posting of this site is beneficial to our suport by spreading article posts of this site to social media marketing accounts which you have such as for example Facebook, Instagram and others or can also bookmark this blog page.
Where does an alpha particle get this symbol.
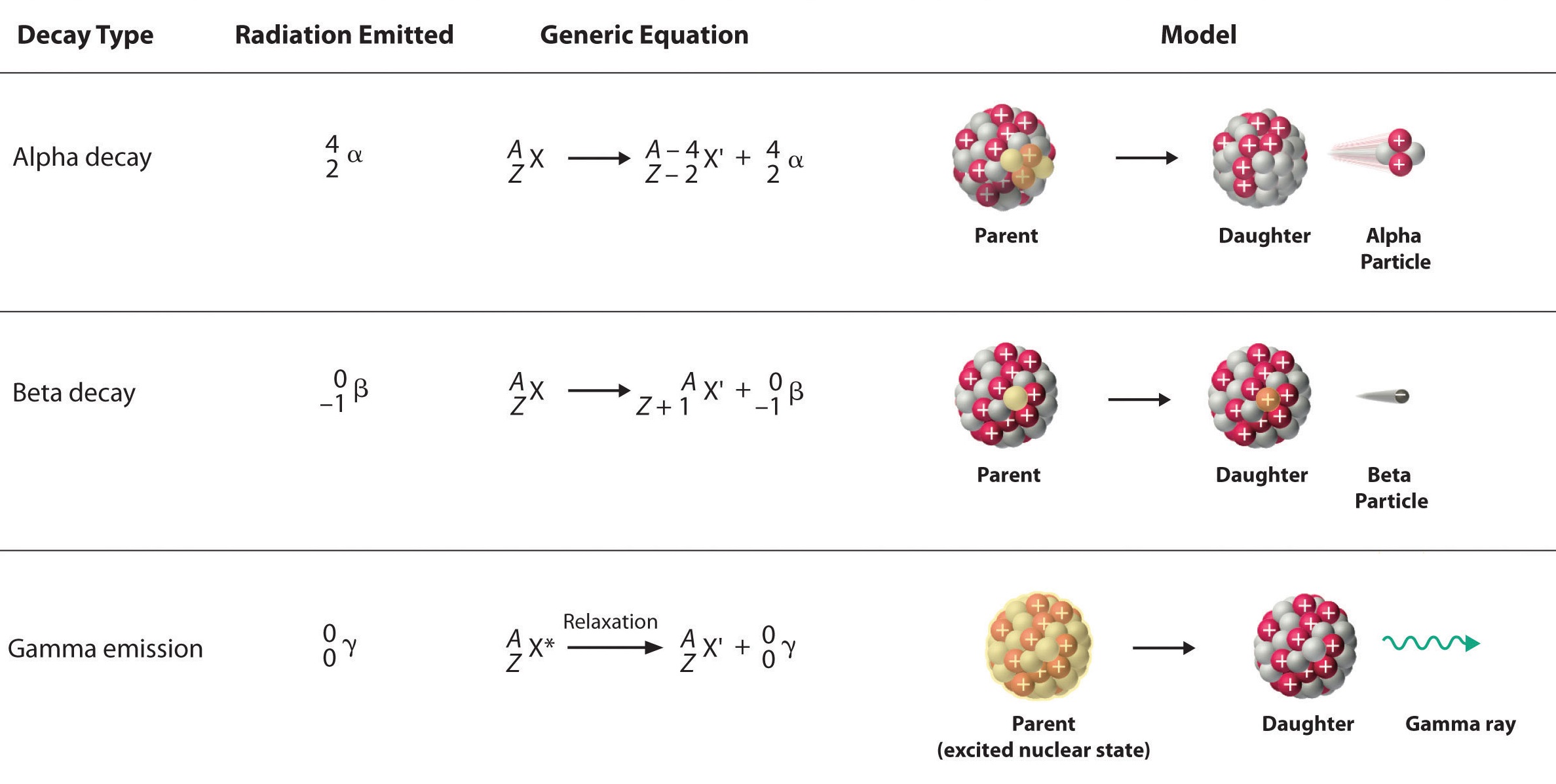
Bohemian grove queen elizabeth. Type of radiatio n nature of the radiation mass 4 amu alpha charge 2 nuclea r symbol a penetrating power low stopped by thin examples americium 241 sheet of paper 4 mass 0 beta gamma b medium most stopped charge 1 by a few mm of metals like aluminum mass 0 very high most stopped charge 0 carbon 14 by a. But what exactly are they. That means that the alpha particle has two protons in it which were lost by the uranium atom.
Look at the symbol for the alpha particle. Especially energetic alpha particles except artificially accelerated helium nuclei are produced in. Alpha particles are relatively large and carry a double positive charge.
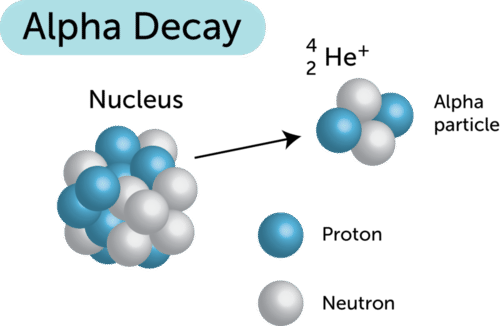
Alpha particles also called alpha rays or alpha radiation consist of two protons and two neutrons bound together into a particle identical to a helium 4 nucleusthey are generally produced in the process of alpha decay but may also be produced in other waysalpha particles are named after the first letter in the greek alphabet athe symbol for the alpha particle is a or a 2. Gamma radiations are high energy radiations that are in the form of electromagnetic waves and these radiations do not give off any particle like alpha and gamma. Alpha particles are commonly emitted by all of the heavy radioactive nuclei occuring in the nature uranium thorium or radium as well as the transuranic elements neptunium plutonium or americium.
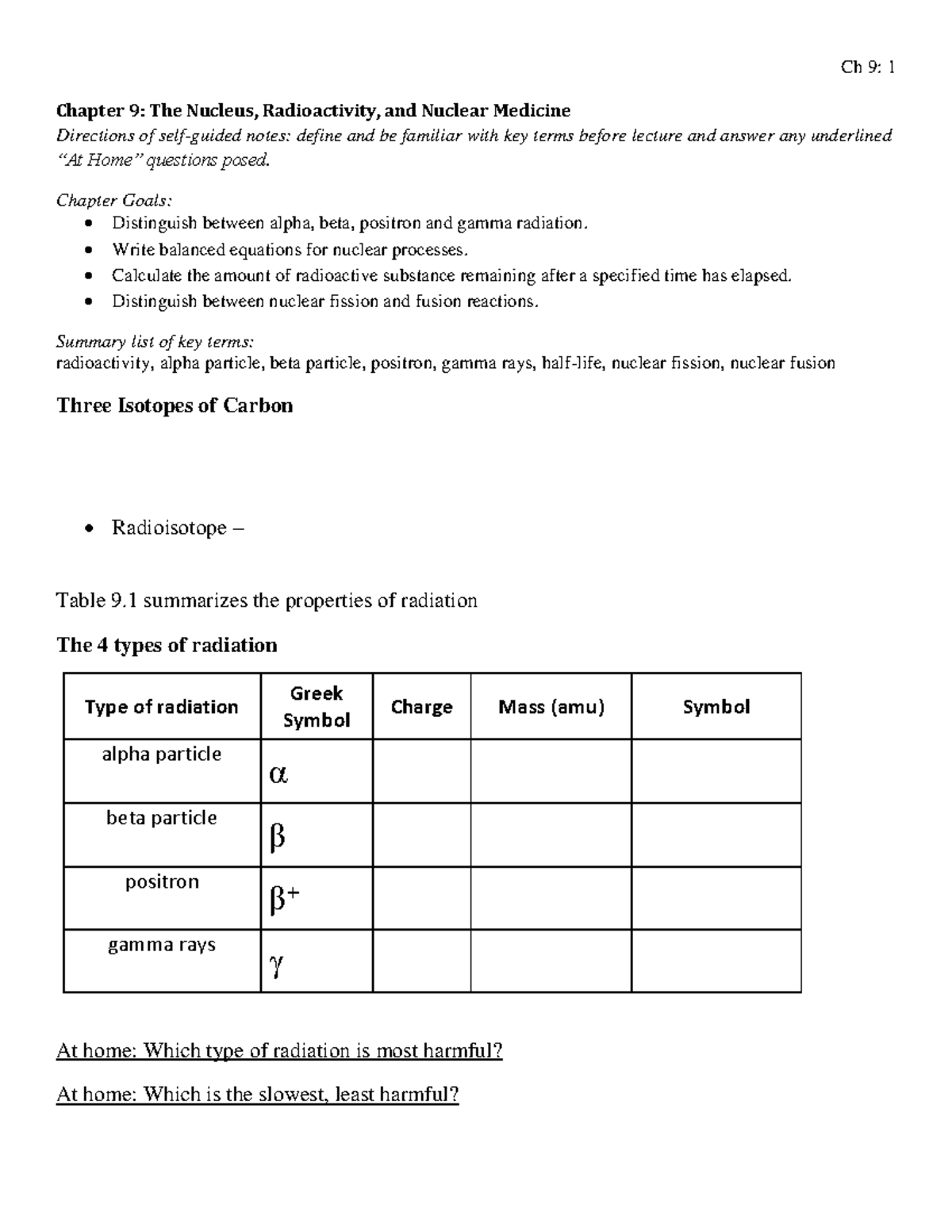
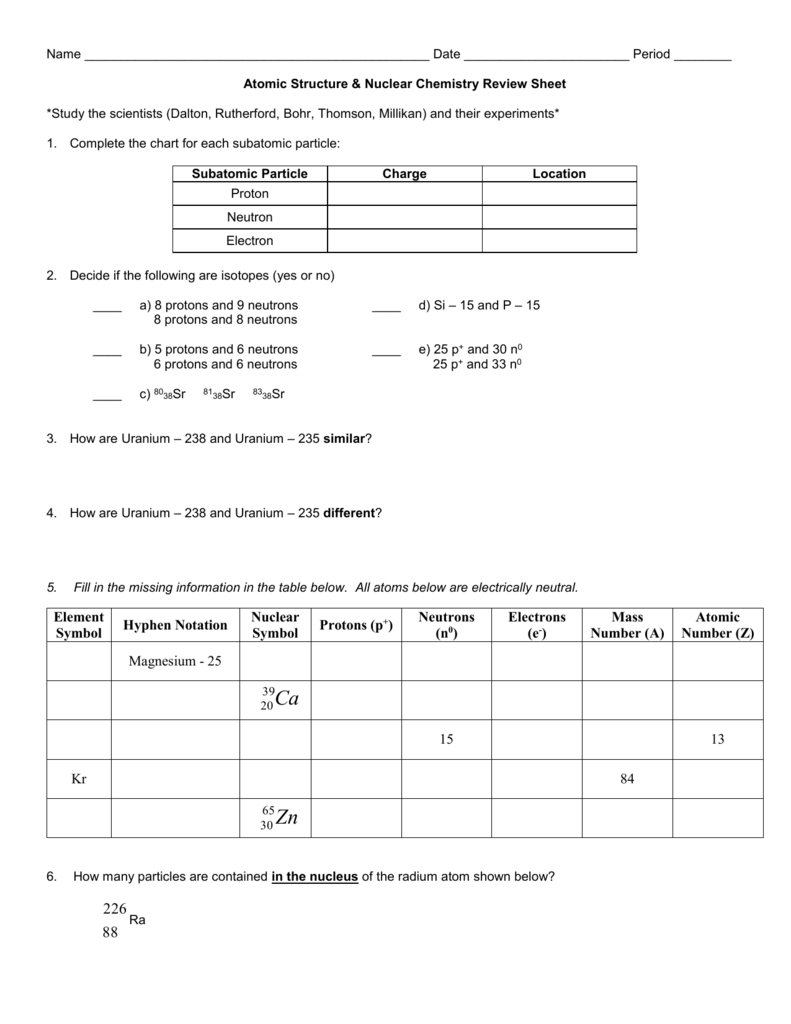
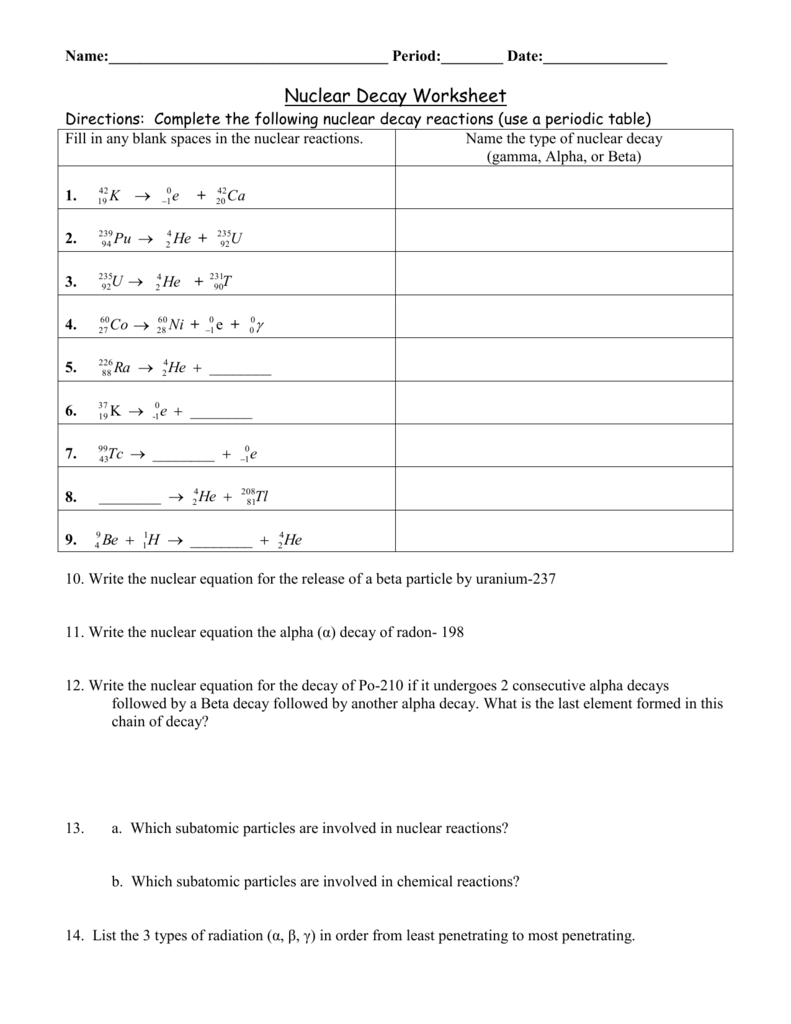
The alpha particle is released along with the kinetic energy it has. Beta radiation is the producer of fast moving electrons and can penetrate further in comparison to the alpha particles. The bottom number in a nuclear symbol is the number of protons.
Alpha radiation consist of alpha particles that are energetic nuclei of heliumthe production of alpha particles is termed alpha decay. The three types of nuclear radiation refer to alpha beta and gamma radiation. In order to become stable a nucleus may emit an alpha particle a helium nucleus or a beta particle an electron or a positron.
Alpha particles consist of two protons and two neutrons bound together into a particle identical to a helium nucleus. Nuclear radiation refers to processes whereby unstable nuclei become more stable by emitting energetic particles. Alpha radiation can be described as the producer of high energy and fast moving helium particles.
In nuclear reactors they are produced for example in the fuel alpha decay of heavy nuclei. The alpha decay symbol. Alpha particles are considered to be a form of dangerous radiation a result of radioactive decay and can cause significant damage to human tissue.
The nucleus is much more stable when the neutrons and protons are eliminated.