Nuclear Reaction Uranium 238
Nuclear Reaction Uranium 238, Indeed recently has been hunted by consumers around us, perhaps one of you personally. People now are accustomed to using the internet in gadgets to view video and image information for inspiration, and according to the name of this article I will discuss about
If the posting of this site is beneficial to our suport by spreading article posts of this site to social media marketing accounts which you have such as for example Facebook, Instagram and others or can also bookmark this blog page.
238 u belongs to primordial nuclides because its half life is comparable to the age of the earth 4510 9 years.

Illegal weapon 20 mp3 download paw. Auranium 238 pb 206 alpha beta note this is a simplified over all reaction the actual process involves around 15 stepsathe equation for the alpha decay of 238u is. If it is hit by a neutron it turns into uranium. Natural uranium is approximately 9927 u 238 072 u 235 and 00055 u 234.
238 u cannot support a chain reaction because. For its very long half life it is still present in. Each of these is produced artificially in a nuclear reactor from the fertile nuclei th 232 in certain reactors u 238 and pu 240 respectively.
Unlike uranium 235 it is non fissile which means it cannot sustain a chain reaction in a thermal neutron reactorhowever it is fissionable by fast neutrons and is fertile meaning it can be transmuted to fissile plutonium 239. Nuclear chain reaction occurs when one single nuclear reaction causes an average of one or more subsequent nuclear reactions thus leading to the possibility of a self propagating series of these reactions. In order to make the fuel uranium is mined and goes through refining and enrichment before being loaded into a nuclear reactor.
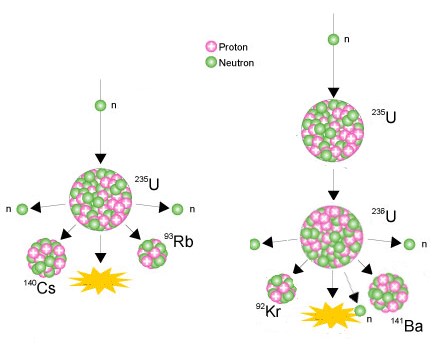
The specific nuclear reaction may be the fission of heavy isotopes eg uranium 235 235 u. U 235 is the only naturally occurring isotope which is thermally fissile and it is present in natural uranium at a concentration of 07. It is an unstable citation needed isotope which accounts for approximately 99284 of naturally occurring uranium isotopesit is also used in warfare for depleted uranium materials such as artillery ammunition and tank armor.
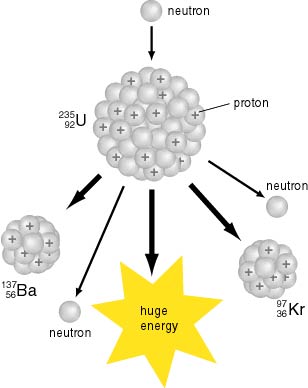
Uranium 238 which alone constitutes 9928 of natural uranium is the most common isotope of uranium in the naturethis isotope has the longest half life 44710 9 years and therefore its abundance is so high. The nuclear chain reaction releases several million times more energy per reaction than any. Because higher concentrations of u 235 are necessary for fission in nuclear reactors and weapons natural uranium is enriched in u 235.
Uranium is the main fuel for nuclear reactors and it can be found in many places around the world. 92238u colorwhitel 90234th 24he note that the sum of the subscripts atomic numbers or charges is the same on each side of the equation. Nuclear fuel pellets with each pellet not much larger than a sugar cube contains as much energy as a tonne.
Uranium 238 also known as depleted uranium and 238 u is the most common uranium isotope that is naturally occurring. Also the sum of the superscripts masses is the same on each side of the equation. Du is uranium primarily composed of the isotope uranium 238 u 238.