Nuclear Reaction Of Uranium 238
Nuclear Reaction Of Uranium 238, Indeed recently has been hunted by consumers around us, perhaps one of you personally. People now are accustomed to using the internet in gadgets to view video and image information for inspiration, and according to the name of this article I will discuss about
If the posting of this site is beneficial to our suport by spreading article posts of this site to social media marketing accounts which you have such as for example Facebook, Instagram and others or can also bookmark this blog page.
Uranium 238 which alone constitutes 9928 of natural uranium is the most common isotope of uranium in the naturethis isotope has the longest half life 44710 9 years and therefore its abundance is so high.
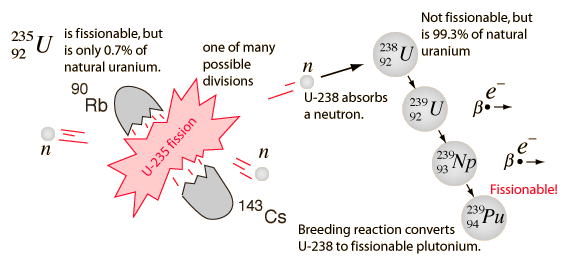
Sleuth anthony shaffer pdf. The standard atomic weight of natural uranium is 238028913. Unlike uranium 235 it is non fissile which means it cannot sustain a chain reaction in a thermal neutron reactorhowever it is fissionable by fast neutrons and is fertile meaning it can be transmuted to fissile plutonium 239. The nuclear chain reaction releases several million times more energy per reaction than any.
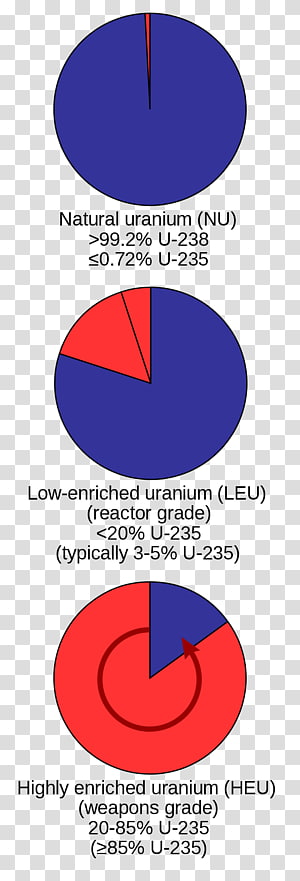
The uranium 235 isotope has a half life of 71 10 8 yr and an abundance of 0711 while the uranium 238 isotope has a half life of 451 10 9 yr and an abundance of 99283. Practically all the naturally occurring atoms of uranium on earth are the uranium 235 and uranium 238 isotopes. The equation for this nuclear reaction is.
Uranium 92u is a naturally occurring radioactive element that has no stable isotopes but two primordial isotopes uranium 238 and uranium 235 that have long half life and are found in appreciable quantity in the earths crust along with the decay product uranium 234. Nuclear chain reaction occurs when one single nuclear reaction causes an average of one or more subsequent nuclear reactions thus leading to the possibility of a self propagating series of these reactions. 238 u belongs to primordial nuclides because its half life is comparable to the age of the earth 4510 9 years.
Usually the emission is not written with atomic number and weight indicated since it is a common particle whose properties should be memorized. Its a physical characteristic of the isotope based on ratio of neutrons to protons in the core and atomic weight. Which is produced from the alpha decay of uranium 238.
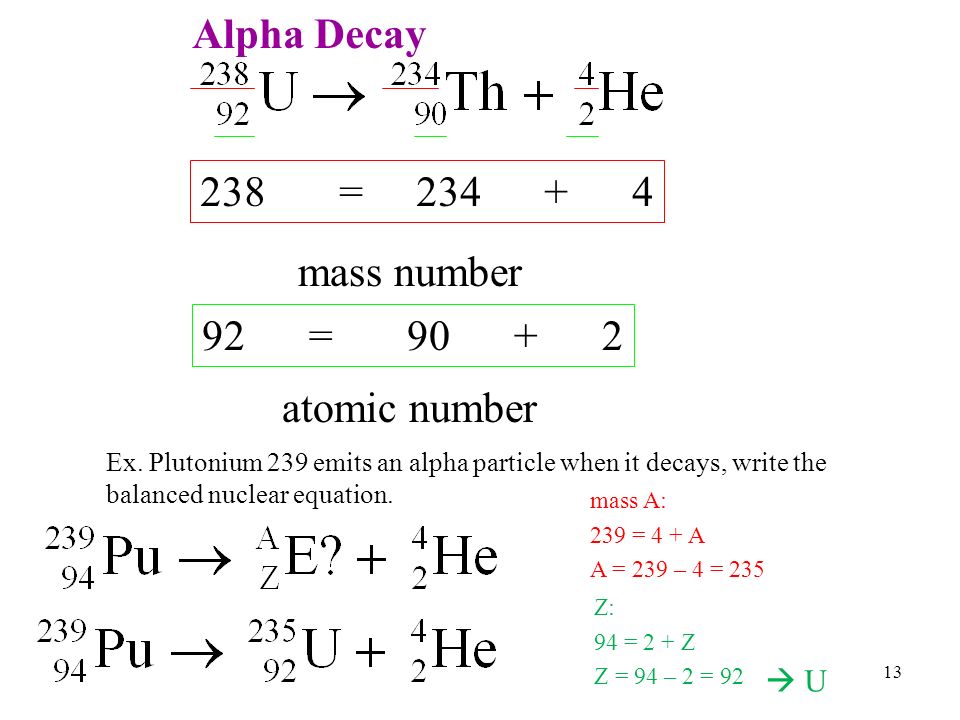
For its very long half life it is still present in. The standard unit for measuring the likelihood of a neutron coming into an atom interacting with the nucleus is a specific way is. Auranium 238 pb 206 alpha beta note this is a simplified over all reaction the actual process involves around 15 stepsathe equation for the alpha decay of 238u is.
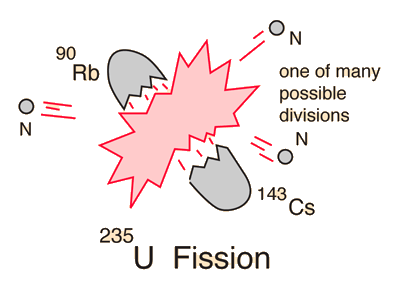
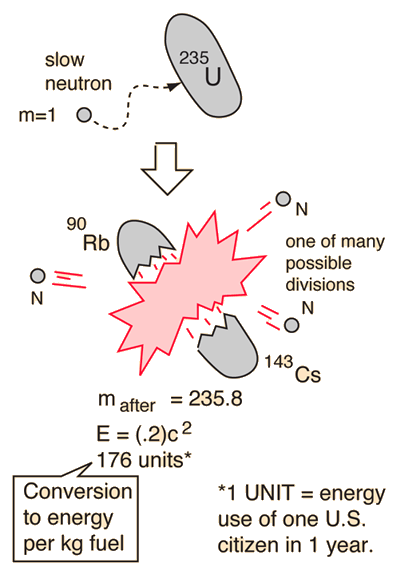
Uranium 238 238 u or u 238 is the most common isotope of uranium found in nature with a relative abundance of 99. The specific nuclear reaction may be the fission of heavy isotopes eg uranium 235 235 u. During a nuclear reaction such as a fission or fusion reaction the mass accounted for by the nuclear binding energy is released in accordance with the equation e mc 2.