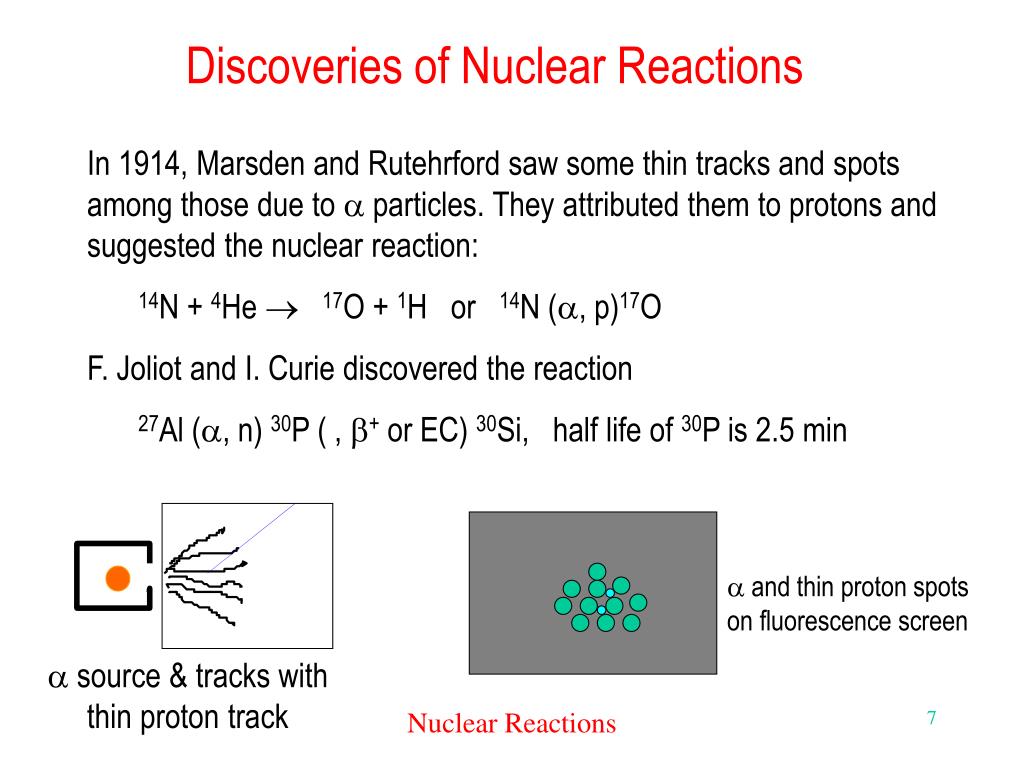
Nuclear Reaction Of Mendelevium
Nuclear Reaction Of Mendelevium, Indeed recently has been hunted by consumers around us, perhaps one of you personally. People now are accustomed to using the internet in gadgets to view video and image information for inspiration, and according to the name of this article I will discuss about
If the posting of this site is beneficial to our suport by spreading article posts of this site to social media marketing accounts which you have such as for example Facebook, Instagram and others or can also bookmark this blog page.

Mendelevium Elizabethtown College S Pictorial Periodic Table Nuclear Energy Henry Moore Nuclear Weapons Korean War
Its monatomic form h is the most abundant chemical substance in the universe constituting roughly 75 of all baryonic mass.

Nuclear energy henry moore nuclear weapons korean war. Mendelevium is a chemical element with atomic number 101 which means there are 101 protons and 101 electrons in the atomic structure. Isotope mass da half life mode of decay nuclear spin nuclear magnetic moment. Atomic mass of mendelevium is 258 u.
Atomic number of mendelevium. There are 16 known radioisotopes ranging in atomic mass from 245 md to 260 md and 5 isomers. Mendelevium atoms have 101 electrons and the shell structure is 2818323182.
Mendelevium 101 md is a synthetic element and thus a standard atomic weight cannot be given. The chemical symbol for mendelevium is md. Characteristics of nuclear fission reaction.
A balanced nuclear reaction equation indicates that there is a rearrangement during a nuclear reaction but of subatomic particles rather than atoms. A metallic radioactive transuranic element in the actinide series it is the first element by atomic number that currently cannot be produced in macroscopic quantities through neutron bombardment of lighter elements. The chemical symbol for mendelevium is md.
With a standard atomic weight of circa 1008 hydrogen is the lightest element on the periodic table. Definition of nuclear fission reaction. Most of the heavier isotopes of the elements with z82 show spontaneous fission.
Further data for naturally occuring isotopes of mendelevium are listed above. Nuclear reactions also follow conservation laws and they are balanced in two ways. This table gives information about some radiosotopes of mendelevium their masses their half lives their modes of decay their nuclear spins and their nuclear magnetic moments.
It is the third to last actinide and the ninth transuranic element. Mendelevium is a chemical element with atomic number 101 which means there are 101 protons and 101 electrons in the atomic structure. Atomic mass of mendelevium.
The chemical symbol for mendelevium is md. Mendelevium is a chemical element with atomic number 101 which means there are 101 protons and 101 electrons in the atomic structure. Note that each element may contain more isotopes therefore this resulting atomic mass is calculated from naturally.
Like all artificial elements it has no stable isotopesthe first isotope to be synthesized was 256 md which was also the first isotope of any element produced one atom at a time in 1955. Nuclear fission is the nuclear process by which a heavy nucleus splits into two or more lighter nuclides of intermediate mass number with release of a large amount of energy. Mendelevium is a synthetic element with the symbol md formerly mv and atomic number 101.
Mendelevium is a metallic radioactive transuranic element in the actinide series it is the first element that currently cannot be produced in macroscopic quantities.

Webelements Periodic Table Mendelevium Properties Of Free Atoms Nuclear Energy Henry Moore Nuclear Weapons Korean War