Nuclear Reaction Formula
Nuclear Reaction Formula, Indeed recently has been hunted by consumers around us, perhaps one of you personally. People now are accustomed to using the internet in gadgets to view video and image information for inspiration, and according to the name of this article I will discuss about
If the posting of this site is beneficial to our suport by spreading article posts of this site to social media marketing accounts which you have such as for example Facebook, Instagram and others or can also bookmark this blog page.
Http Sarsunacollege Ac In Webpages Downloads Elearning Science Physics Ug 204th 20sem Fission And Fusion Teacher Notes Pdf Nuclear Reactor Uranium China Buat Matahari
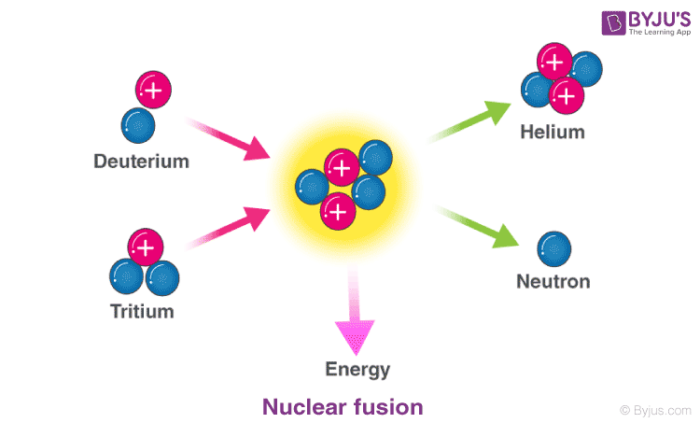
The vast energy potential of nuclear fusion was first exploited in thermonuclear.

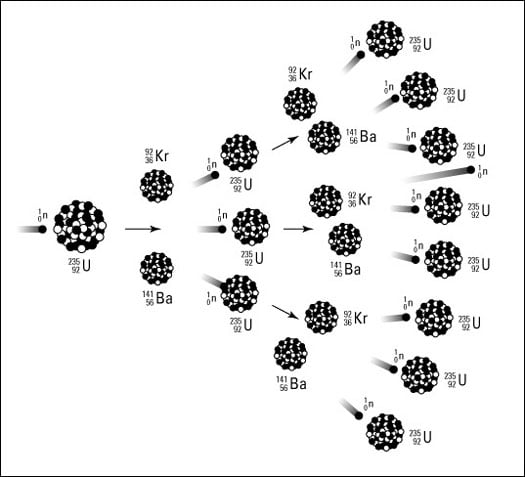
Nuclear reactor uranium china buat matahari. A typical nuclear reaction is depicted in figure 171. The two general kinds of nuclear reactions are nuclear decay reactions and nuclear transmutation reactionsin a nuclear decay reaction also called radioactive decay an unstable nucleus emits radiation and is transformed into the nucleus of one or more other elementsthe resulting daughter nuclei have a lower mass and are lower in energy more stable than the parent nucleus that decayed. This equation describes neutron capture in the boron which is diluted in the coolant.
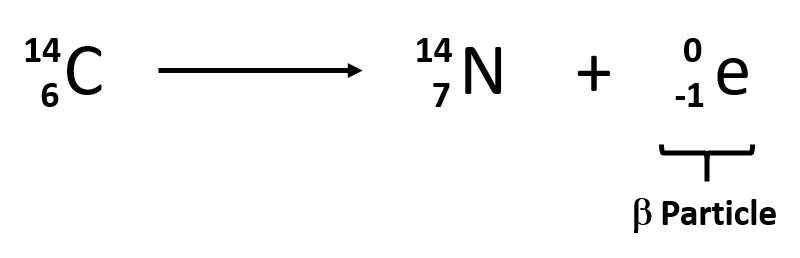
It is the contrary reaction of fission where heavy isotopes are split apart. A b r c. An example of this notation follows.
During a nuclear reaction such as a fission or fusion reaction the mass accounted for by the nuclear binding energy is released in accordance with the equation e mc 2 energy mass times the square of the speed of light. Nuclear reactions also follow conservation laws and they are balanced in two ways. In the centre of mass frame where a and b are the initial species about to collide c is the final species and r is the resonant state.
The following two ways of describing that reaction are equivalent. A balanced nuclear reaction equation indicates that there is a rearrangement during a nuclear reaction but of subatomic particles rather than atoms. Nuclear reactions may be shown in a form similar to chemical equations for which invariant mass must balance for each side of the equation and in which transformations of particles must follow certain conservation laws such as conservation of charge and baryon number total atomic mass number.
The sum of the mass numbers of the reactants equals the sum of the mass numbers of the products. Instead of using the full equations in the style above in many situations a compact notation is used to describe nuclear reactions. Nuclear fusion process by which nuclear reactions between light elements form heavier elements up to iron.
Fusion is the means by which the sun and other stars generate light and heat. The above reaction is the kind we shall focus on because they represent most of the important. Ax yb or xaby.
The following apply for the nuclear reaction. Nuclear fusion is a nuclear process where the energy is generated by smashing together light atoms. Boric acid is used in nuclear power plants as a long term compensator of nuclear fuel reactivity.
In cases where the interacting nuclei belong to elements with low atomic numbers eg hydrogen atomic number 1 or its isotopes deuterium and tritium substantial amounts of energy are released. Nuclear reactions may be shown in a form similar to chemical equations for which invariant mass which is the mass not considering the mass defect must balance for each side of the equation.