Nuclear Reaction Equations Examples
Nuclear Reaction Equations Examples, Indeed recently has been hunted by consumers around us, perhaps one of you personally. People now are accustomed to using the internet in gadgets to view video and image information for inspiration, and according to the name of this article I will discuss about
If the posting of this site is beneficial to our suport by spreading article posts of this site to social media marketing accounts which you have such as for example Facebook, Instagram and others or can also bookmark this blog page.
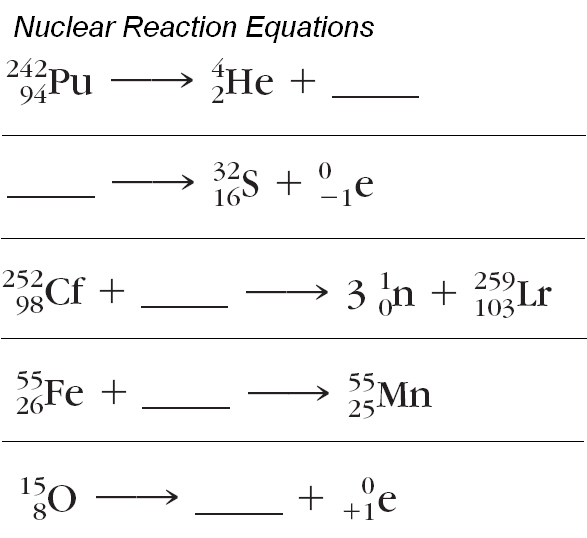
Instead of using the full equations in the style above in many situations a compact notation is used to describe nuclear reactions.
Bohemian grove location google earth. The reaction in our example above would be written as li 6daa. An important example of nuclear fission is the splitting of the uranium 235 nucleus when it is bombarded with neutrons. Notation of nuclei source.
The equation for this nuclear reaction is. In these examples the sum of the masses top and the sum of the proton numbers bottom are the same on. Nuclear reactions also follow conservation laws and they are balanced in two ways.
Balancing equations for nuclear reactions the reaction of an a particle with magnesium 25 latex1225textmglatex produces a proton and a nuclide of another element. Solution the nuclear reaction can be written as. This equation describes neutron capture in the boron which is diluted in the coolant.
Balancing equations for nuclear reactions the reaction of an a particle with magnesium 25 12 25 mg 12 25 mg produces a proton and a nuclide of another element. The two general kinds of nuclear reactions are nuclear decay reactions and nuclear transmutation reactionsin a nuclear decay reaction also called radioactive decay an unstable nucleus emits radiation and is transformed into the nucleus of one or more other elementsthe resulting daughter nuclei have a lower mass and are lower in energy more stable than the parent nucleus that decayed. Identify the new nuclide produced.
Various products can be formed from this nuclear reaction as described in the equations below. A balanced nuclear reaction equation indicates that there is a rearrangement during a nuclear reaction but of subatomic particles rather than atoms. Boric acid is used in nuclear power plants as a long term compensator of nuclear fuel reactivity.
Nuclear reactions also follow conservation laws and they are balanced in two ways. The sum of the mass numbers of the reactants equals the sum of the mass numbers of the products. Solution the nuclear reaction can be written as.
In balancing a nuclear equation it is important to remember that the sum of all the mass numbers and atomic numbers given on the upper left and lower left side of the element symbol respectively must be equal for both sides of the. Nuclear equations represent the reactants and products in radioactive decay nuclear fission or nuclear fusion. Instead of chemical equations where it shows the different number of elements is conserved in a reaction in a nuclear reaction the atomic mass and proton number are conserved.
Identify the new nuclide produced.