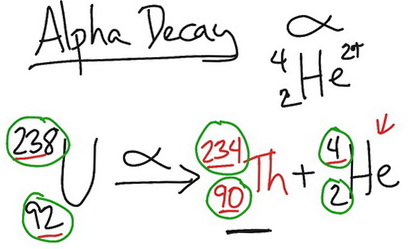Nuclear Reaction Alpha Decay
Nuclear Reaction Alpha Decay, Indeed recently has been hunted by consumers around us, perhaps one of you personally. People now are accustomed to using the internet in gadgets to view video and image information for inspiration, and according to the name of this article I will discuss about
If the posting of this site is beneficial to our suport by spreading article posts of this site to social media marketing accounts which you have such as for example Facebook, Instagram and others or can also bookmark this blog page.
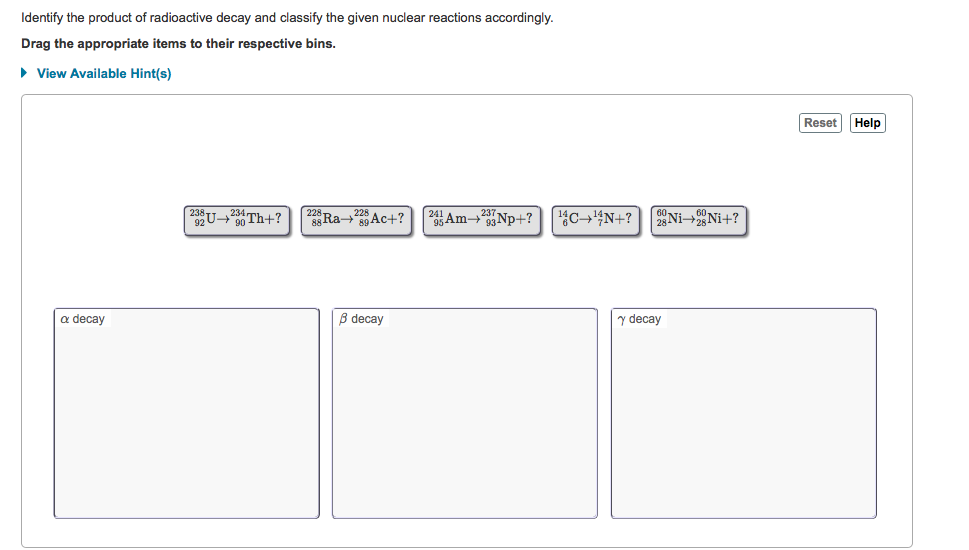
Solved Identify The Product Of Radioactive Decay And Clas Chegg Com Hiroshima Nuclear Radiation Today
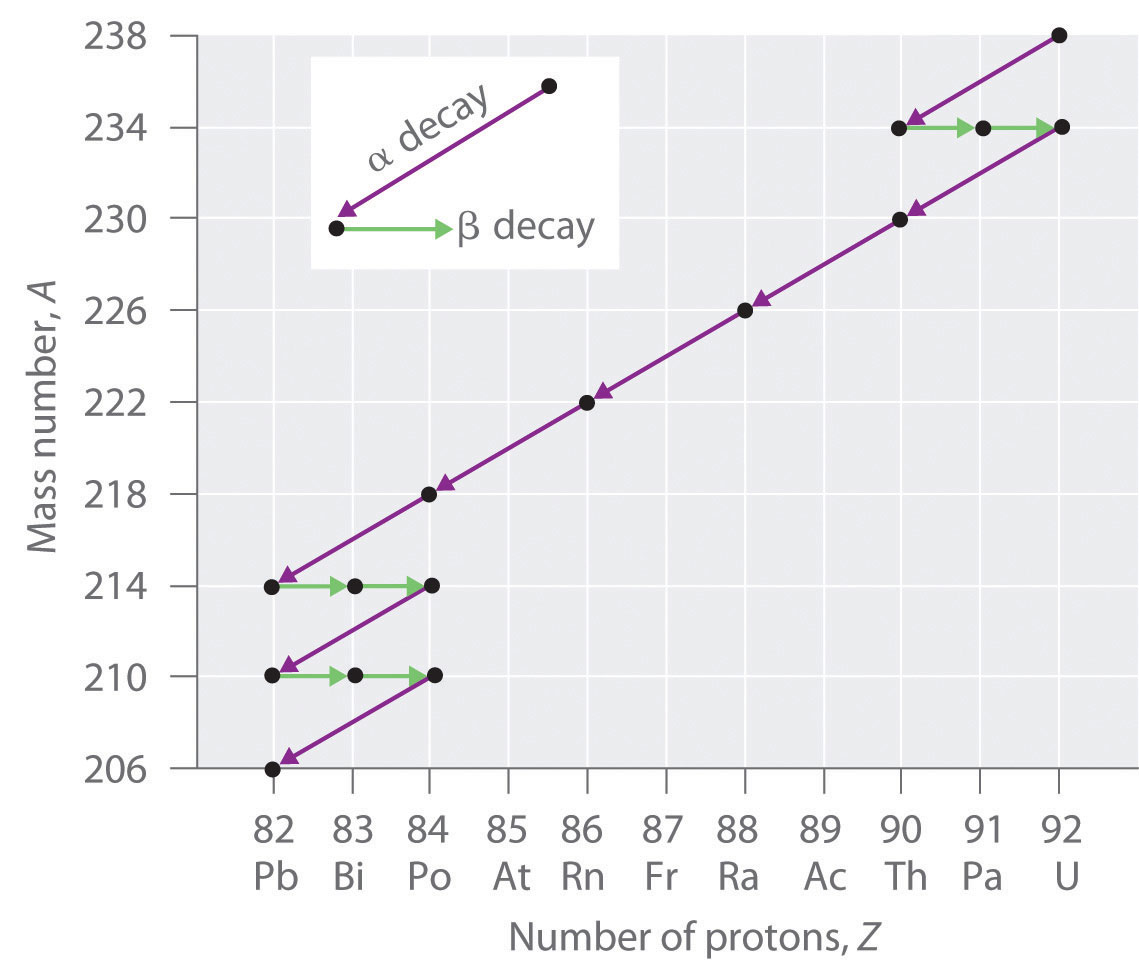
The number of protons must also be consistent on both sides of the reaction.

Hiroshima nuclear radiation today. Other important types of nuclear reactions alpha decay. The atomic numbers and mass numbers in a nuclear equation must be balanced. Alpha decay fission and nuclear reactions alpha decay fission and nuclear reactions energy release in alpha decay consider a nucleus which is stable against decay by proton or neutron emission the least bound nucleon still has say several mev of binding energy.
Introduction to nuclear engineering 2nd edition. Find the decay of a nuclear reaction examples of nuclear reactions st 90 decay graph example of gamma rays in chemistry nuclerar reactions tutorials gamma decay example beta positive decay neutron increase by one gamma decay of 55137cs which of the following is true for a nuclear reaction beta positive decay gamma decay rates of alpha radiation. In alpha decay the unstable isotope will emit an alpha particle along with a more stable isotope or isotopes.
The alpha decay of platinum 175 in this reaction platinum 175 undergoes a decay to produce osmium 171. Protons and neutrons are made up of quarks. The masses of the elements are conserved during alpha decay.
Energy including rest mass energy is conserved in nuclear reactions. This was the first observation of an induced nuclear reaction that is a reaction in which particles from one decay are used to transform another atomic nucleus. The general equation for alpha decay is.
Alpha decay q value. Nuclei with mass numbers greater than 200 tend to undergo alpha decay a process in which a 4 he nucleus commonly referred to as an alpha particle 4 2 a is liberated from the parent nucleus. In 1919 ernest rutherford was able to accomplish transmutation of nitrogen into oxygen at the university of manchester using alpha particles directed at nitrogen 14 n a 17 o p.
The alpha particle is the same as a helium nucleus with 2 protons and 2 neutrons. Alpha decay occurs when the nucleus of an atom spontaneously ejects an alpha particle. In nuclear and particle physics the energetics of nuclear reactions is determined by the q value of that reaction.
A z x a 4 z 2 x 4 2 a. Most nuclear reactions emit energy in the form of gamma rays. Nuclear reactions need to have the sum of protons and neutrons the same on both sides of the equation.
A nuclear reaction is one that changes the structure of the nucleus of an atom.



.svg.png?revision=1)