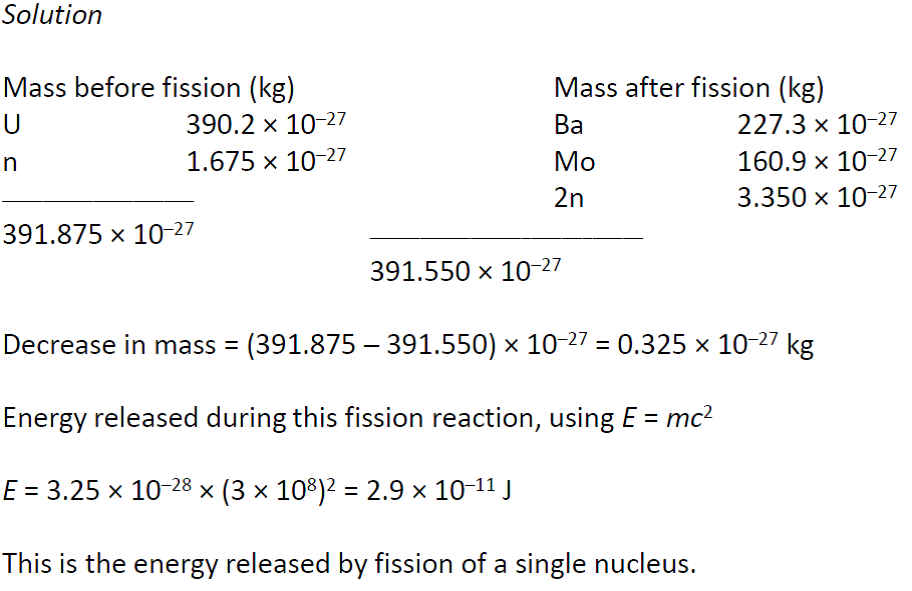Nuclear Fusion Reaction Equations
Nuclear Fusion Reaction Equations, Indeed recently has been hunted by consumers around us, perhaps one of you personally. People now are accustomed to using the internet in gadgets to view video and image information for inspiration, and according to the name of this article I will discuss about
If the posting of this site is beneficial to our suport by spreading article posts of this site to social media marketing accounts which you have such as for example Facebook, Instagram and others or can also bookmark this blog page.
Fusing two light nuclei can liberate as much energy as the fission of 235 u or 239 pu.

Nuclear fusion of deuterium and tritium nuclear power no thanks. That is problematic because it is harder to extract the energy from neutrons compared to charged particles. If nuclear fusion could be produced in a commercial setting it could provide 3 4 times the energy a fission reaction generates. During a nuclear reaction such as a fission or fusion reaction the mass accounted for by the nuclear binding energy is released in accordance with the equation e mc 2 energy mass times the square of the speed of light.
A following the method used in example 2161 calculate the change in mass that accompanies the reaction. B calculate the change in mass per mole of 235 u. The sum of the mass numbers of the reactants equals the sum of the mass numbers of the products.
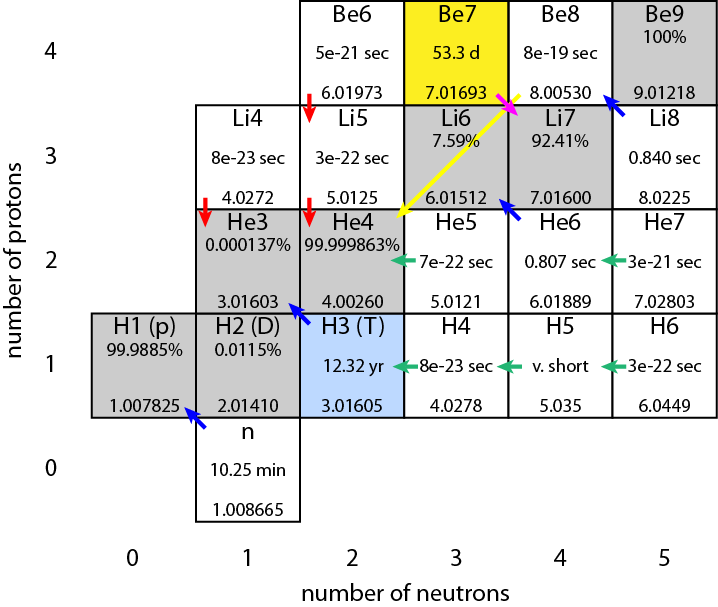
Nuclear fusion process by which nuclear reactions between light elements form heavier elements up to iron. Balanced nuclear reaction asked for. Although a fusion reaction generates more energy than a fission reaction modern nuclear power plants utilize fission processes due to the stability of the fission reaction convenience and cost of production.
The graph of binding energy per nucleon suggests another way of obtaining useful energy from nuclear reactions. In cases where the interacting nuclei belong to elements with low atomic numbers eg hydrogen atomic number 1 or its isotopes deuterium and tritium substantial amounts of energy are released. Then use equation 2163 to calculate the change.
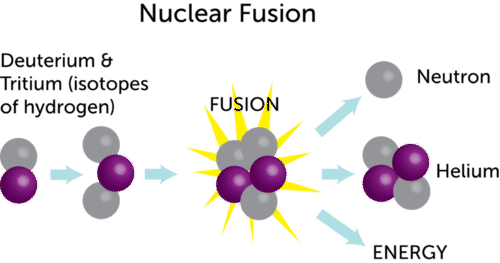
Nuclear fusion is a reaction in which two or more atomic nuclei are combined to form one or more different atomic nuclei and subatomic particles neutrons or protonsthe difference in mass between the reactants and products is manifested as either the release or the absorption of energythis difference in mass arises due to the difference in atomic binding energy between the nuclei before and. Energy released in electronvolts per atom and kilojoules per mole strategy. Fusion reactions occur in stars where two hydrogen nuclei fuse together under high temperatures and pressure.
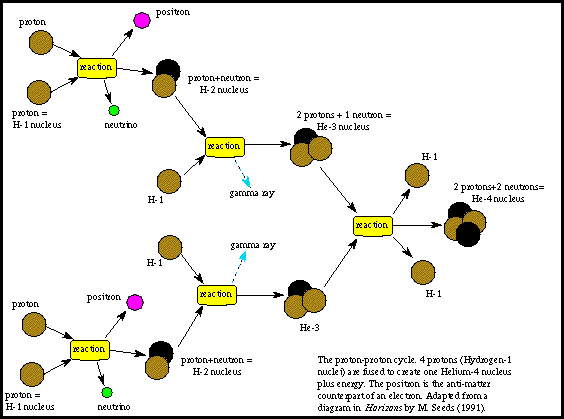
A balanced nuclear reaction equation indicates that there is a rearrangement during a nuclear reaction but of subatomic particles rather than atoms. The vast energy potential of nuclear fusion was first exploited in thermonuclear. The fusion of four protons to form a helium nucleus two positrons and two neutrinos for example generates 247 mev of energy.
Nuclear fusion is when two small light nuclei join together to make one heavy nucleus. Nuclear reactions also follow conservation laws and they are balanced in two ways. Convert this value to the change in energy in electronvolts per atom.

2 3 Nuclear Reactions Physics Hyndland Nuclear Fusion Of Deuterium And Tritium Nuclear Power No Thanks