Nuclear Decay Reaction Equation
Nuclear Decay Reaction Equation, Indeed recently has been hunted by consumers around us, perhaps one of you personally. People now are accustomed to using the internet in gadgets to view video and image information for inspiration, and according to the name of this article I will discuss about
If the posting of this site is beneficial to our suport by spreading article posts of this site to social media marketing accounts which you have such as for example Facebook, Instagram and others or can also bookmark this blog page.
These processes are quite similar to nuclear reactions but are spontaneous rather than induced.
Nuclear reaction analysis ppt. Balancing a radioactive decay equation. Nuclear decay a process through which an unstable nucleus emits radiation in order to lose energy. Spontaneous fission reactions nuclear fission reactions that do not require a neutron to proceed and are therefore not induced.
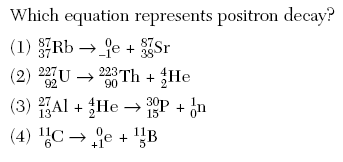
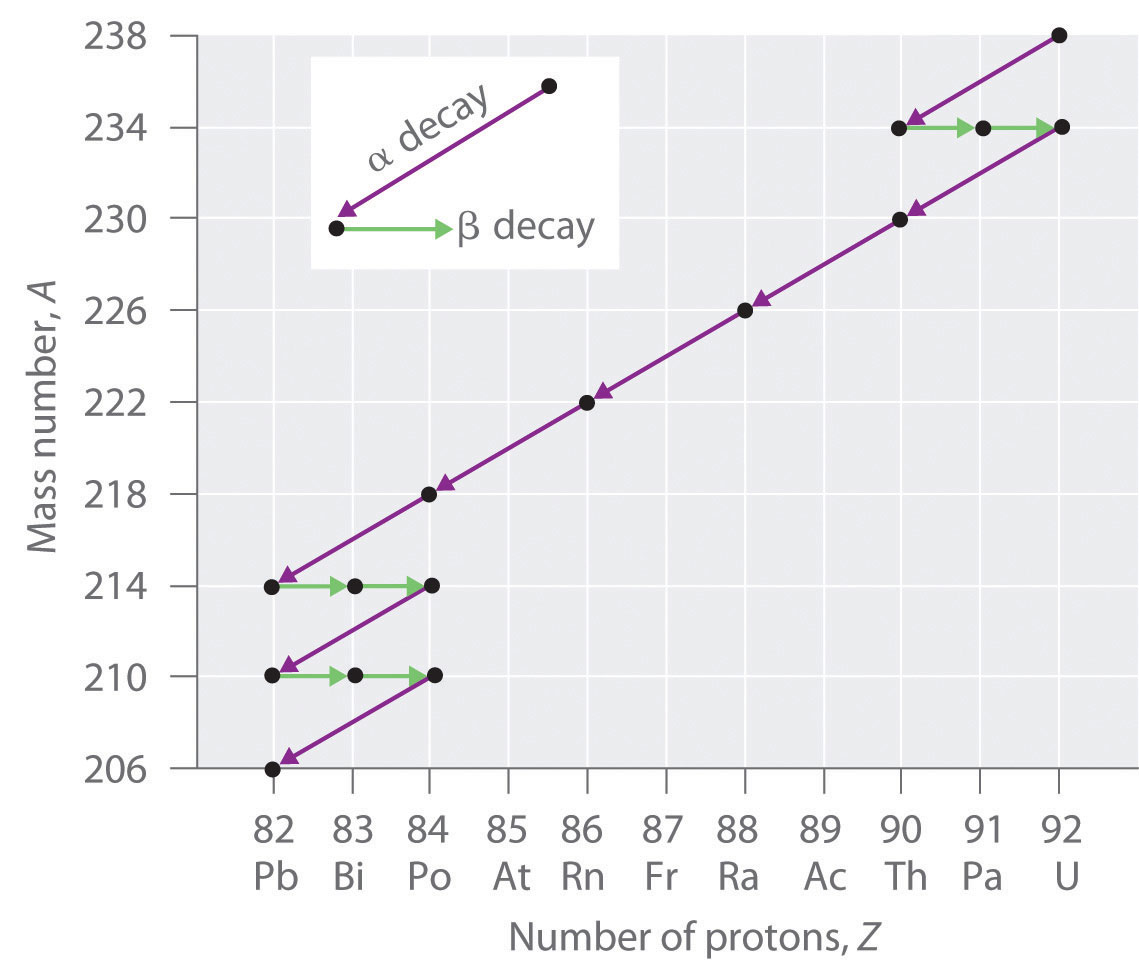
Radioactive decay also known as nuclear decay radioactivity radioactive disintegration or nuclear disintegration is the process by which an unstable atomic nucleus loses energy by radiationa material containing unstable nuclei is considered radioactivethree of the most common types of decay are alpha decay beta decay and gamma decay all of which involve emitting one or more particles. In 1919 ernest rutherford was able to accomplish transmutation of nitrogen into oxygen at the university of manchester using alpha particles directed at nitrogen 14 n a 17 o p. The reaction in our example above would be written as li 6daa.
The two general kinds of nuclear reactions are nuclear decay reactions and nuclear transmutation reactionsin a nuclear decay reaction also called radioactive decay an unstable nucleus emits radiation and is transformed into the nucleus of one or more other elementsthe resulting daughter nuclei have a lower mass and are lower in energy more stable than the parent nucleus that decayed. This constant is called the decay constant and is denoted by l lambda. The radioactive decay law states that the probability per unit time that a nucleus will decay is a constant independent of time.
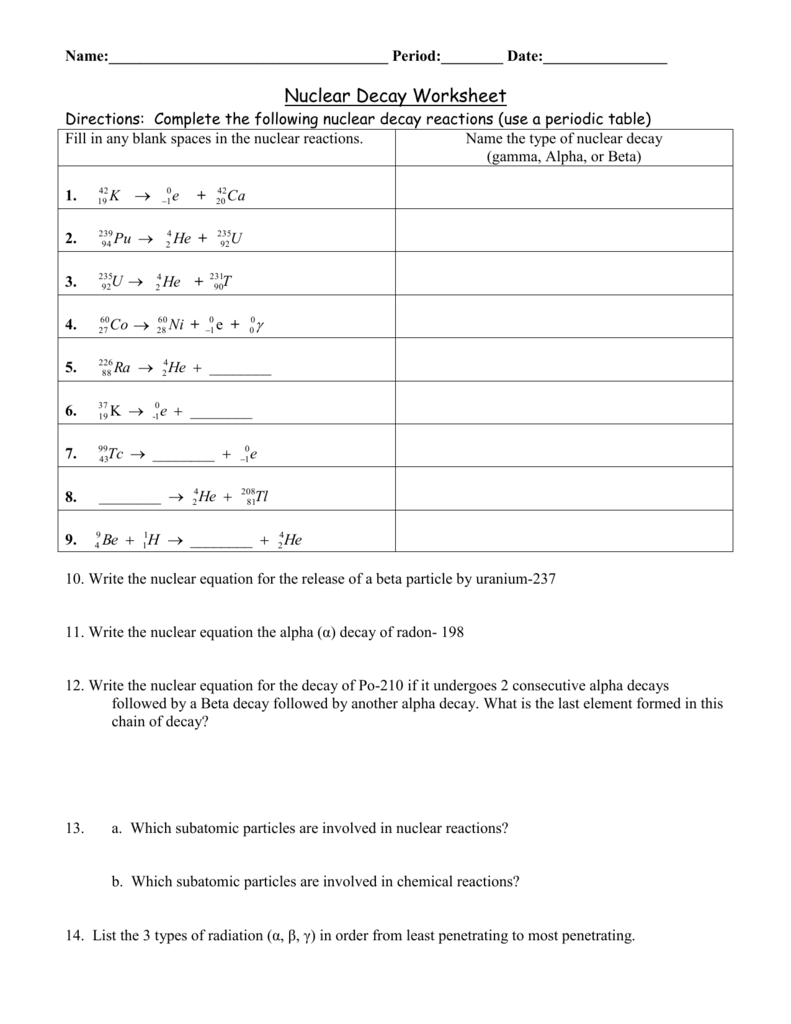
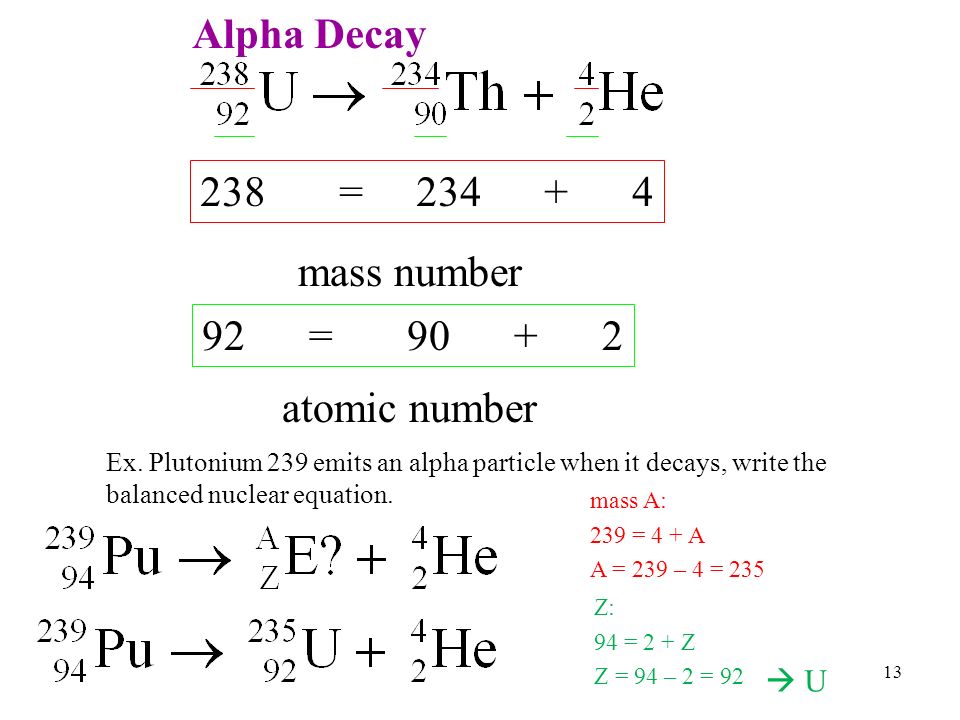
This was the first observation of an induced nuclear reaction that is a reaction in which particles from one decay are used to transform another atomic nucleus. In balancing a nuclear equation it is important to remember that the sum of all the mass numbers and atomic numbers given on the upper left and lower left side of the element symbol respectively must be equal for both sides of the. A nuclear reaction is considered to be the process in which two nuclear particles two nuclei or a nucleus and a nucleon interact to produce two or more nuclear particles or rays thus a nuclear reaction must cause a transformation of at least one nuclide to another.
A nuclear decay equation shows the nucleus of the unstable isotope parent on the left hand side of the equation and the type of radiation emitted as well as the new nucleus daughter is shown on the right hand side of the equation. Sometimes if a nucleus interacts with another nucleus or particle without changing the nature of any nuclide the process.