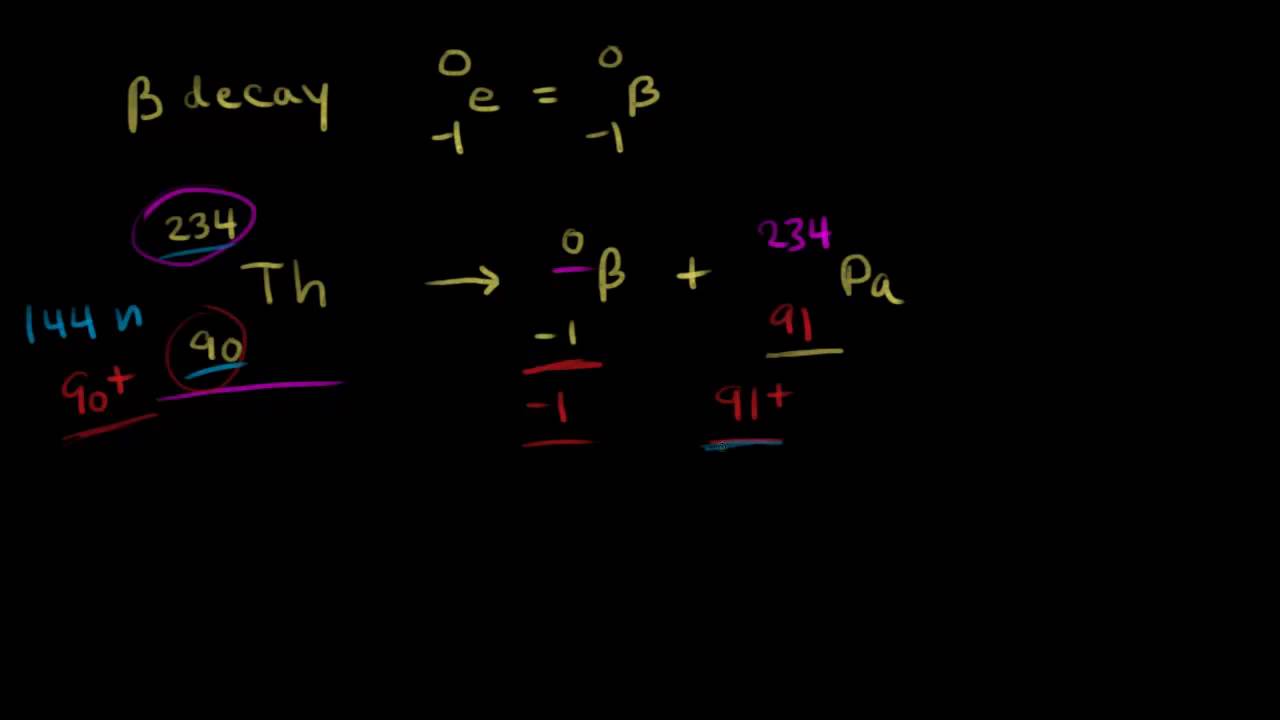Alpha Radiation Nuclear Equation
Alpha Radiation Nuclear Equation, Indeed recently has been hunted by consumers around us, perhaps one of you personally. People now are accustomed to using the internet in gadgets to view video and image information for inspiration, and according to the name of this article I will discuss about
If the posting of this site is beneficial to our suport by spreading article posts of this site to social media marketing accounts which you have such as for example Facebook, Instagram and others or can also bookmark this blog page.

Nuclear Equations Worksheet Answers Typepad Pages 1 3 Flip Pdf Download Fliphtml5 What Is Nuclear Energy Name The Process Used For Producing Electricity Using The Nuclear Energy China Nuclear You Tube

Balancing Nuclear Equations Ppt Video Online Download What Is Nuclear Energy Name The Process Used For Producing Electricity Using The Nuclear Energy China Nuclear You Tube
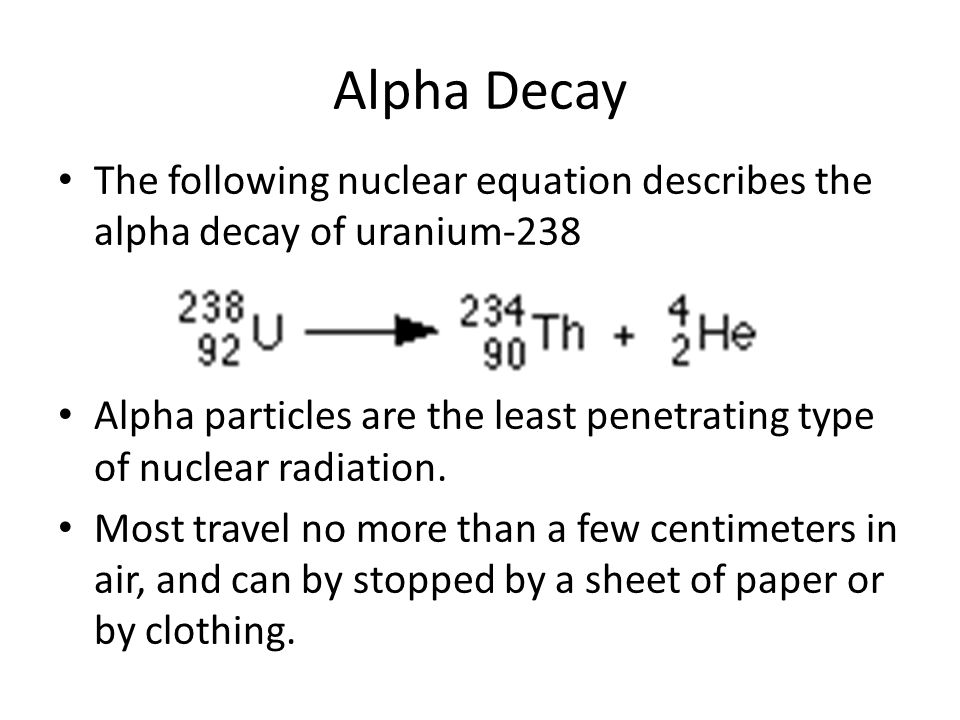
The atomic number decreases by 2.

What is nuclear energy name the process used for producing electricity using the nuclear energy china nuclear you tube. An alpha particle is the. The atomic mass number decreases by 4. In 1899 ernest rutherford wrote the following words.
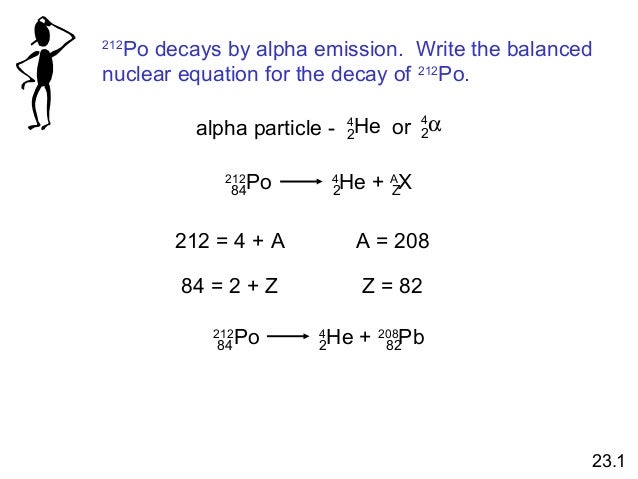
So for representing an alpha particle in our nuclear equation since an alpha particle has the same composition as a helium nucleus we put an he in here and it has two positive charges so we put a two down here and then a total of four nucleons so we put a four here. Alpha particles are relatively large and carry a double positive charge. Alpha radiation consist of alpha particles that are energetic nuclei of heliumthe production of alpha particles is termed alpha decay.
Alpha particles are commonly emitted by all of the heavy radioactive nuclei occuring in the nature uranium thorium or radium as well as the transuranic elements neptunium plutonium or americium. The atomic numbers and mass numbers in a nuclear equation must be balanced. Especially energetic alpha particles except artificially accelerated helium nuclei are produced in.
Absorbed dose total ionizing dose total energy of radiation transferred to unit mass d can only be found experimentally na gy 1 jkg gray l 2 t 2. Most nuclear reactions emit energy in the form of gamma rays. Protons and neutrons are made up of quarks.
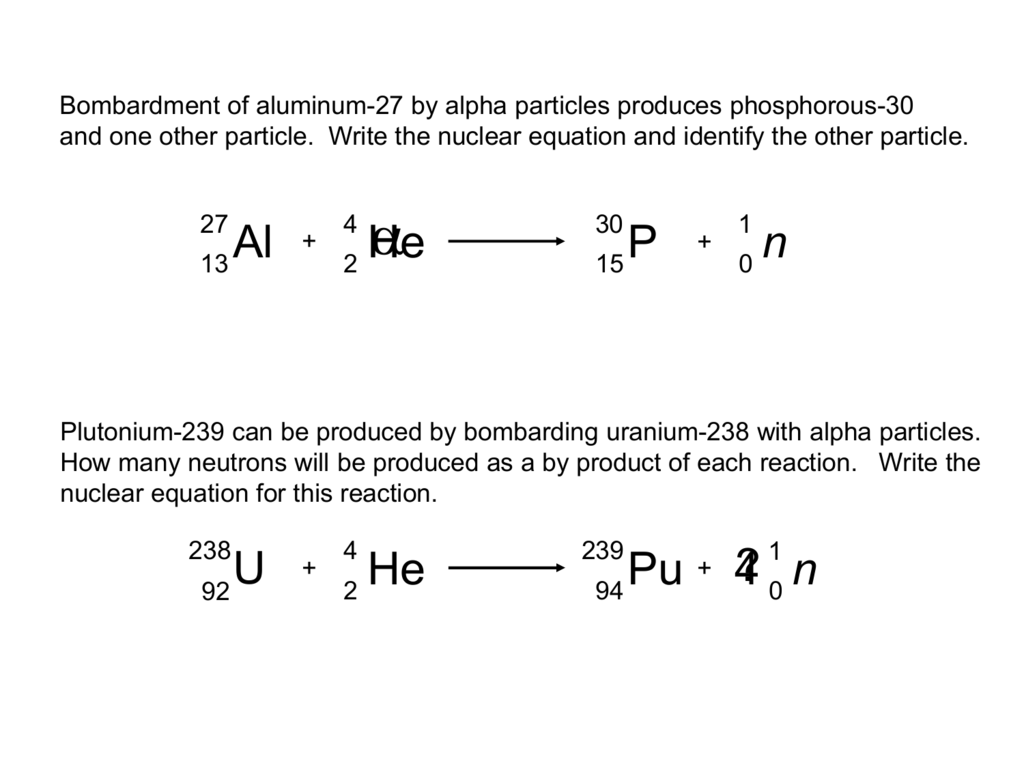
In nuclear reactors they are produced for example in the fuel alpha decay of heavy nuclei. List of equations in nuclear and particle physics jump to. Alpha decay two protons and two neutrons changes the mass number of the element by 4 and the atomic number by 2.
These changes are described using nuclear equations. A nuclear reaction is one that changes the structure of the nucleus of an atom. Alpha particles consist of two protons and two neutrons bound together into a particle identical to a helium nucleus.
The subscripts and superscripts are necessary for balancing nuclear equations but are usually optional in other circumstances. Two protons and two neutrons are lost from a nucleus when it emits an alpha particlethis means that. For example an alpha particle is a helium nucleus he with a charge of 2 and a mass number of 4 so it is symbolized latex24texthelatex.
These experiments show that the uranium radiation is complex and that there are present at least two distinct types of radiation one that is very readily absorbed which will be termed for convenience the alpha radiation and the other of more penetrative character which will be termed the beta radiation.
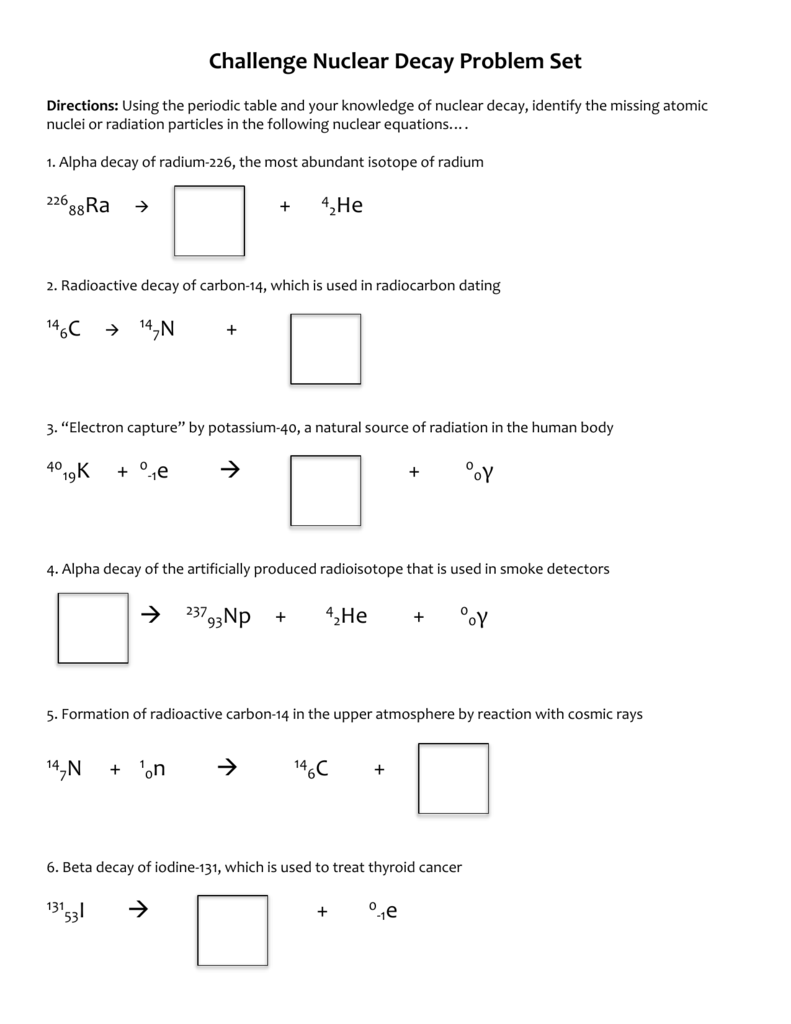
Challenge Nuclear Decay Worksheet What Is Nuclear Energy Name The Process Used For Producing Electricity Using The Nuclear Energy China Nuclear You Tube