Nuclear Reaction Quantities
Nuclear Reaction Quantities, Indeed recently has been hunted by consumers around us, perhaps one of you personally. People now are accustomed to using the internet in gadgets to view video and image information for inspiration, and according to the name of this article I will discuss about
If the posting of this site is beneficial to our suport by spreading article posts of this site to social media marketing accounts which you have such as for example Facebook, Instagram and others or can also bookmark this blog page.

Table 4 1 From Simnra User S Guide Semantic Scholar Nuclear Power Efficiency Comparison World War Four

Solved Chem 12 Your Name Nuclear Chemistry Activity And Chegg Com Nuclear Power Efficiency Comparison World War Four
Activity specific activity.
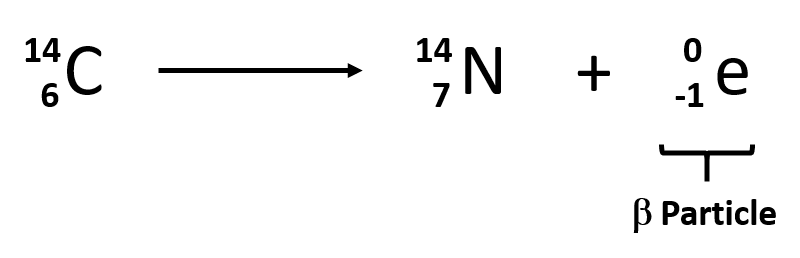
Nuclear power efficiency comparison world war four. 1 mass is conserved as 2 3 5 and 4 1 5 2 charge is conserved as 1 1 2 and 2 0 2. Nuclear reaction change in the identity or characteristics of an atomic nucleus induced by bombarding it with an energetic particle. What are nuclear reactions.
Once mass defect is known nuclear binding energy can be calculated by converting that mass to energy by using emc 2. This chapter briefly summarizes key differences between quantities and units of radiation. The quantities that are conserved in nuclear reaction.
Mass must be in units of kg. Once this energy which is a quantity of joules for one nucleus is known it can be scaled into per nucleon and per mole quantities. When balancing a nuclear reaction.
An example of this kind of a nuclear reaction occurs in the production of cobalt 60 within a nuclear reactor. In nuclear physics and nuclear chemistry a nuclear reaction is semantically considered to be the process in which two nuclei or a nucleus and an external subatomic particle collide to produce one or more new nuclidesthus a nuclear reaction must cause a transformation of at least one nuclide to another. A nuclear reaction is considered to be the process in which two nuclear particles two nuclei or a nucleus and a nucleon interact to produce two or more nuclear particles or rays thus a nuclear reaction must cause a transformation of at least one nuclide to another.
Sometimes if a nucleus interacts with another nucleus or particle without changing the nature of any nuclide the process. What quantities are conserved in nuclear reactions. Well theyre reactions between nuclei.
The cobalt 60 then decays by the emission of a beta particle plus gamma rays into nickel 60this reaction has a half life of about 527 years and due to the availability of cobalt 59 100 of its natural abundance this neutron bombarded isotope of cobalt is a valuable source of. In the above example. The bombarding particle may be an alpha particle a gamma ray photon a neutron a proton or a heavy ion.
Mass number a is the sum of number of protons and neutrons in a nucleus. A measure of radioactivity activity is based on counting of disintegrations per secondthe si unit of activity is the becquerel bq equal to one reciprocal second. To convert to joulesmole simply multiply by avogadros number.
Well the mass number and the charge are the two things that are always conserved in a nuclear reaction in every nuclear reaction. Alright so whats conserved in a nuclear reaction. Learn more about nuclear reactions in this article.
If a nucleus interacts with another nucleus or particle and they then separate without. Mass number is also known as nucleon number. Every single radioactive decay every single nuclear collision every single nuclear reaction.





