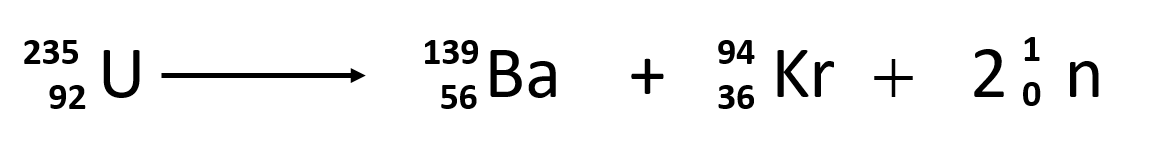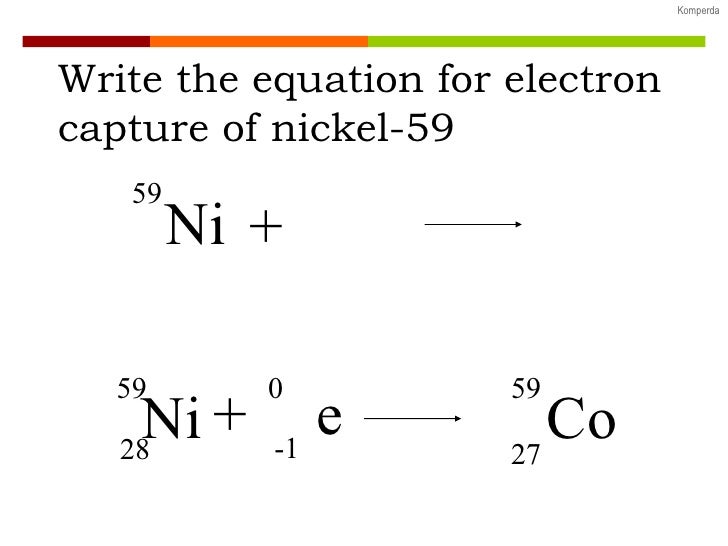Nuclear Reaction For Electron Capture
Nuclear Reaction For Electron Capture, Indeed recently has been hunted by consumers around us, perhaps one of you personally. People now are accustomed to using the internet in gadgets to view video and image information for inspiration, and according to the name of this article I will discuss about
If the posting of this site is beneficial to our suport by spreading article posts of this site to social media marketing accounts which you have such as for example Facebook, Instagram and others or can also bookmark this blog page.
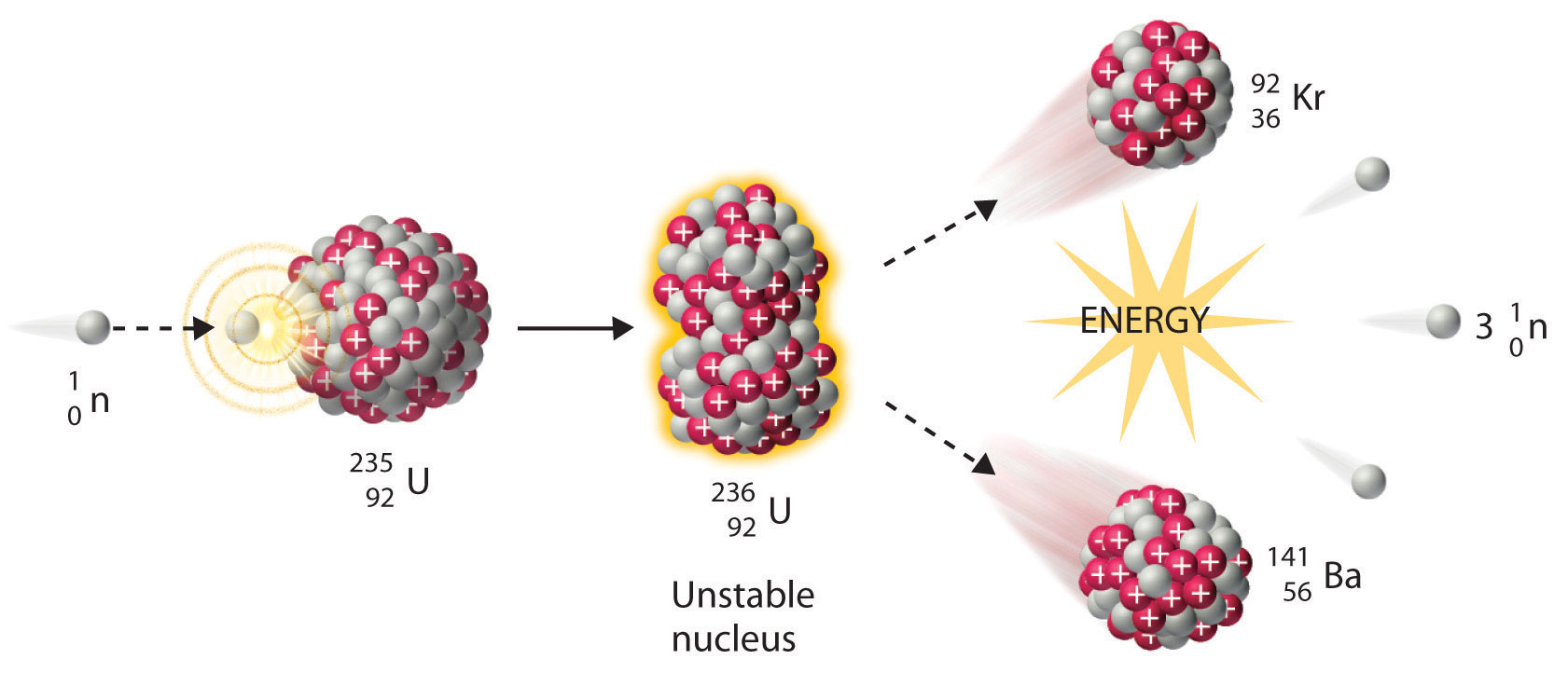
Nuclear reactions may be shown in a form similar to chemical equations.
Nuclear power plants being built sci hub gangguan. Neutron capture type of nuclear reaction in which a target nucleus absorbs a neutron uncharged particle then emits a discrete quantity of electromagnetic energy gamma ray photon. Neutron capture ng reactions are among the more common types of nuclear reactions that involve compound nucleus formation. Thus gamma ray emission and beta decay are the most common occurrences.
This process reduces the atomic number by 1 and emits gamma radiation or an x ray and a neutrino. In most situations involving low energy neutrons the compound nucleus has insufficient energy for nucleon emission. An alternate way for a nuclide to increase its neutron to proton ratio is by a phenomenon called electron capture.
A representing an alpha particle or helium 4 b for beta particle or electron g for gamma photon etc. Fazard from intakes of electron capture nuclides attachment 3 is a table that gives an indication of the relative significance of electron capture nuclides as an internal radiation hazard. Thus phosphorus 31 on undergoing neutron capture becomes phosphorus 32.
Electron capture k electron capture also k capture or l electron capture l capture is a process in which the proton rich nucleus of an electrically neutral atom absorbs an inner atomic electron usually from the k or l electron shellsthis process thereby changes a nuclear proton to a neutron and simultaneously causes the emission of an electron neutrino. The nuclear reaction is written 8 15 o e 7 15 n v a write the process going on for a single particle within the nucleus. The reaction above would be written as 6 lidaa.
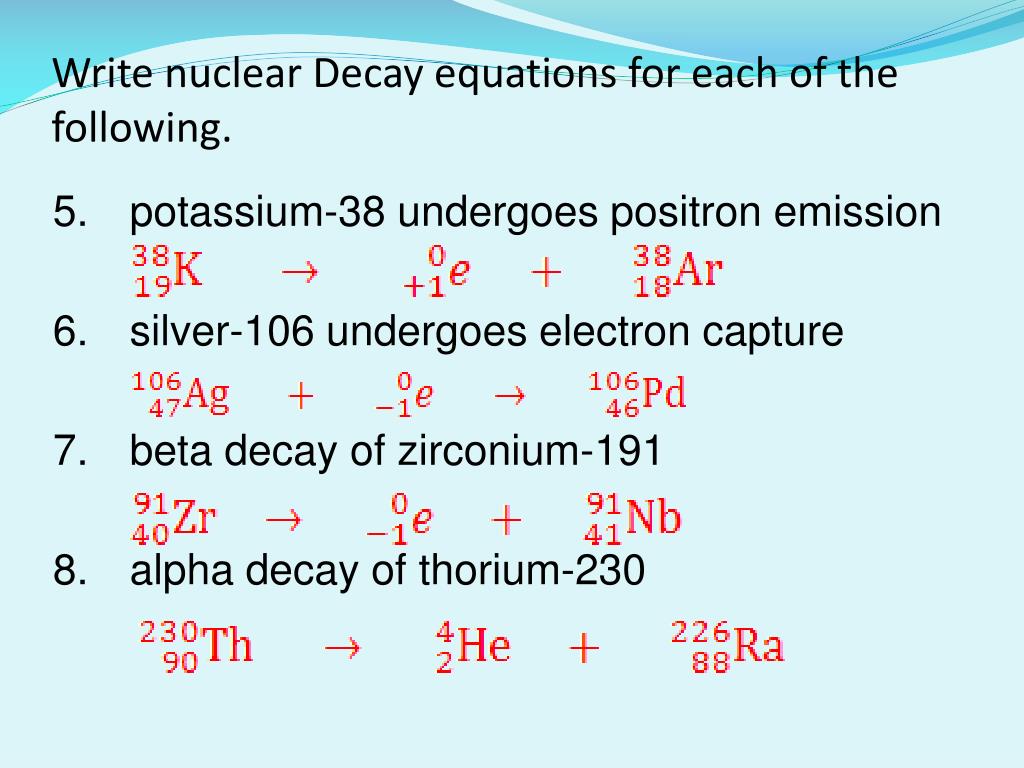
For example silver 106 undergoes electron capture to become palladium 106. Using the argonne tandem linear accelerator system atlas and gammasphere a. The target nucleus and the product nucleus are isotopes or forms of the same element.
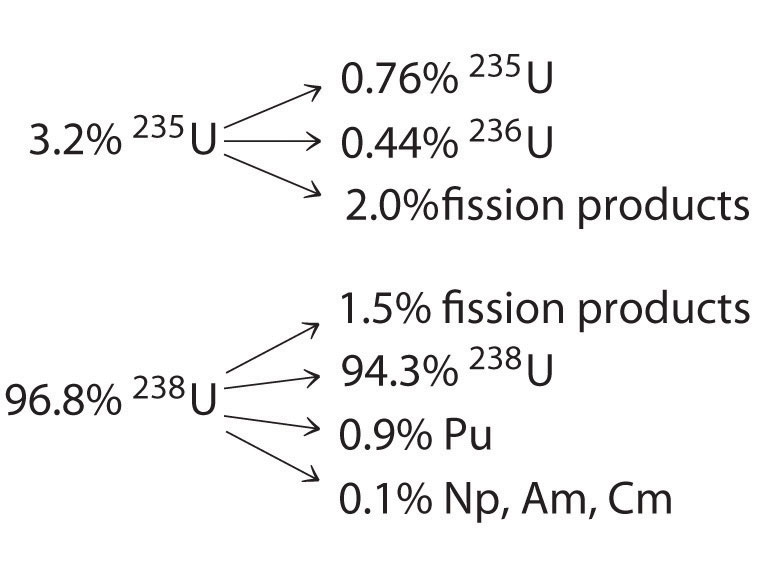
Neutron capture and nuclear fission. B disregarding the daughters recoil determine the energy of the neutrino. Electron capture occurs when an inner orbital electron negatively charged is captured by the nucleus positively charged.
Physicists first predicted the effect called nuclear excitation by electron capture neec over 40 years ago. This is one of the means that a nuclear change can take place. But scientists had not seen it until now.
When a nuclear change takes place an atom changes its identity and is now an atom of a different element. This advance tests theoretical models that describe how nuclear and atomic realms interact and may also provide new insights into how star elements are created. Physics physics for scientists and engineers with modern physics the nucleus 8 15 o decays by electron capture.