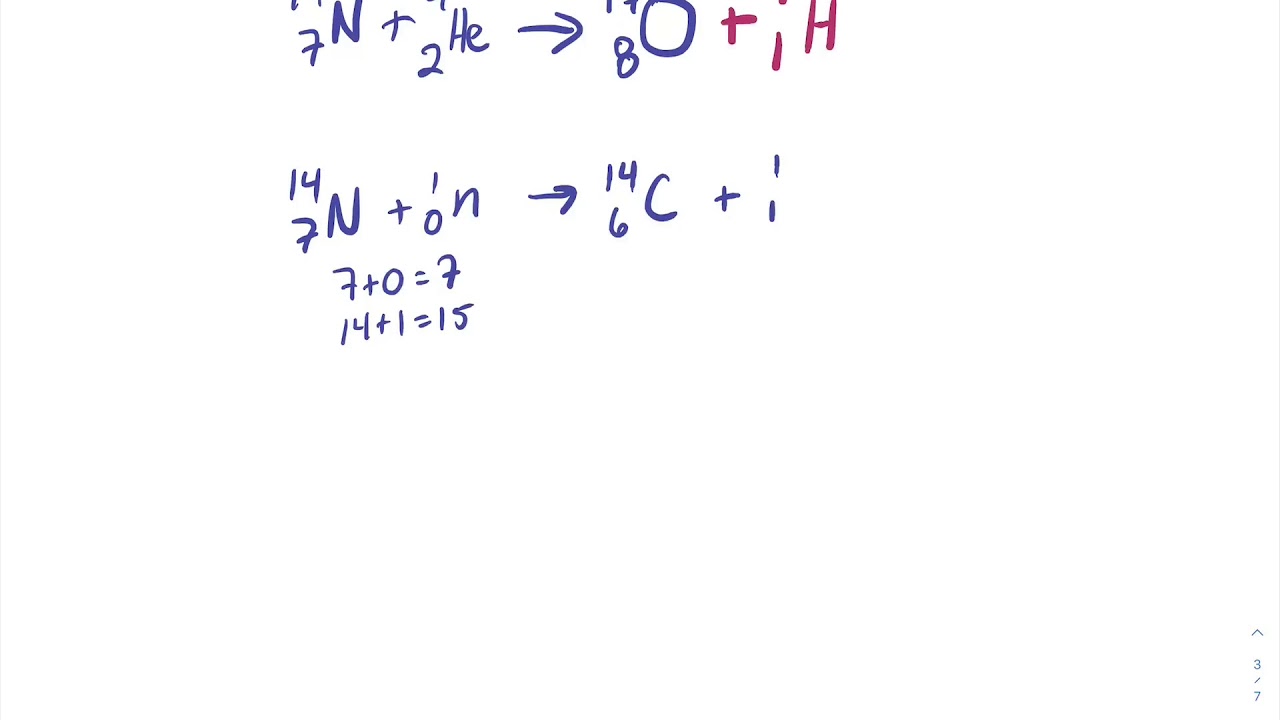Nuclear Reaction For Chromium 51 Decays By Electron Capture
Nuclear Reaction For Chromium 51 Decays By Electron Capture, Indeed recently has been hunted by consumers around us, perhaps one of you personally. People now are accustomed to using the internet in gadgets to view video and image information for inspiration, and according to the name of this article I will discuss about
If the posting of this site is beneficial to our suport by spreading article posts of this site to social media marketing accounts which you have such as for example Facebook, Instagram and others or can also bookmark this blog page.

The Half Lives Of Different Medical Radioisotopes Are Given In The Table If The Initial Amount Of Homeworklib Ordained Minister En Espanol
51 cr is produced in a reactor by neutron activation.
Ordained minister en espanol. It is used to label red blood cells for measurement of mass or volume survival time and sequestration studies for the diagnosis of gastrointestinal bleeding and to label platelets to study their survival. Chromium 51 decays by electron capture. In each of the following nuclear reactions supply the missing particle.
Physics physics for scientists and engineers with modern physics the nucleus 8 15 o decays by electron capture. 51 cr is a useful red cell label and also has utility as a platelet label. Write a balanced equation for the decay process and identify the daughter that is produced.
Now we explain radioactive decays radiation one by one. Rubidium 83 will decay into krypton 83 by electron capture. The capture rates depend sensitively on the distribution of the gamowteller gt strength.
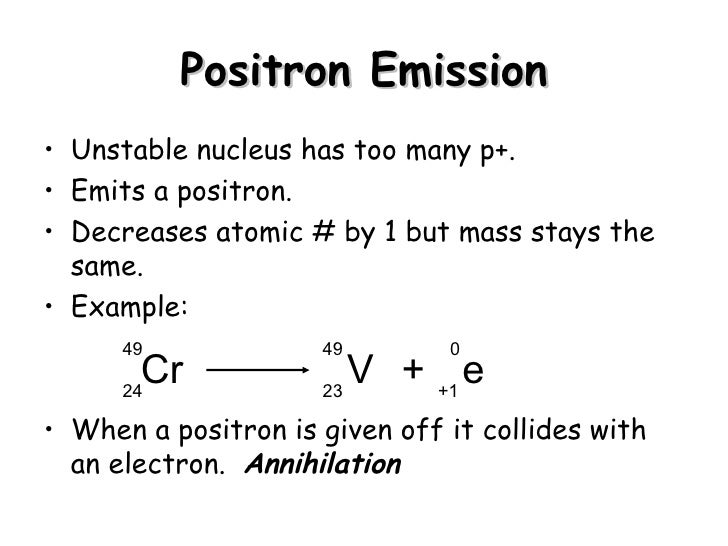
60co 60ni. After alpha decay atomic number. Chromium 51 which targets the spleen and is used as a tracer in studies of red blood cells decays by electron capture.
Natural nuclear reactions and radioactive decays. Electron capture k electron capture also k capture or l electron capture l capture is a process in which the proton rich nucleus of an electrically neutral atom absorbs an inner atomic electron usually from the k or l electron shellsthis process thereby changes a nuclear proton to a neutron and simultaneously causes the emission of an electron neutrino. The nuclear reaction is written 8 15 o e 7 15 n v a write the process going on for a single particle within the nucleus.
Chemistry chemistry for today. It decays by electron capture with a radioactive half life of. Get 11 help now from expert chemistry tutors.
Get more help from chegg. Advantages of this radionuclide include ease of red cell labeling excellent red cell uptake low toxicity and low and stable elution rate. Alpha a particles can be called helium 4 nuclei 2 4 he 2.
In radioactive reactions charge and mas number are conserved. Electron capture is a process in which a proton in the nucleus captures one of the inner electrons and forms a neutron. Natural nuclear reactions and radioactive decays.
Thus the equation for electron capture in the decay of rubidium 83 is. General organic and biochemistry chromium 51 is used medically to monitor kidney activity. Chromium 51 is a synthetic radioactive isotope of chromium having a half life of 277 days and decaying by electron capture with emission of gamma rays 032 mev.
Chromium 51 decays by electron capture. 11h 1e 01n since a proton becomes a neutron the number of protons decreases by 1 but the atomic mass stays the same. B disregarding the daughters recoil determine the energy of the neutrino.
Electron capture reactions on ni and co isotopes are investigated by shell model calculations in steller environments.